Criegee chemistry is captured
DOI: 10.1063/PT.3.1462
The chemistry of the atmosphere is complicated. Anthropogenic and naturally produced molecules undergo thousands of chemical reactions that affect urban pollution and smog, stratospheric ozone depletion, and global climate, among other things. Each reaction has its own kinetic rate constant, which must be known if the system is to be modeled and its effects understood.
Among the key players in atmospheric chemistry is a class of unstable molecules called Criegee intermediates. Although qualitative evidence for their atmospheric role is strong, until recently no one had ever directly detected a Criegee intermediate in the gas phase, let alone measured its rates of reaction with other molecules.
Now, Craig Taatjes and David Osborn (both at Sandia National Laboratories in Livermore, California) and their colleagues have made several direct kinetic measurements on the reactions between the simplest Criegee intermediate, CH2OO, and four small atmospheric molecules: SO2, NO2, NO, and water. 1 The results are particularly relevant to sulfate and nitrate chemistry in the atmosphere.
Mysterious molecules
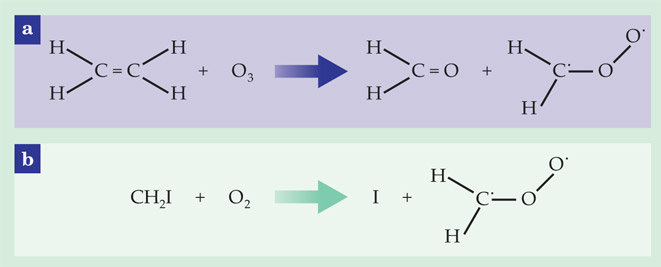
As first suggested by Rudolf Criegee in 1949, the eponymous intermediates form during ozonolysis, the reaction of ozone (O3) with alkenes, or unsaturated hydrocarbons. That reaction is shown in figure 1a. Alkenes and ozone are both abundant in polluted urban environments; so, too, are Criegee intermediates, which then react to form, for example, OH radicals, organic and inorganic acids, and low-volatility organic compounds that condense into aerosol droplets, or smog.

Figure 1. Cooking up Criegee intermediates. (a) In the atmosphere—and in many an organic chemistry lab—Criegee intermediates such as CH2OO form by ozonolysis, the reaction of unsaturated hydrocarbons with ozone. (b) But the reaction of CH2I with O2 is more conducive to studying Criegee kinetics in the laboratory. The dots on the C and O atoms indicate unpaired electrons.
Ozonolysis has been well studied in the liquid phase, where it’s an important tool in the synthetic organic chemistry toolbox. The final products are exactly what are expected, given the Criegee intermediates’ involvement. Studies of ozonolysis products, and how those products change when other reagents are added to the mix, have allowed some estimates of the rates of Criegee reactions. Extracting reaction rates from final-product measurements requires extensive modeling of the whole reaction system, which is no easy task. But until now, it’s all that atmospheric chemists have had to go on.
One challenge in making direct measurements has been in forming enough of the Criegee intermediate and keeping it around for long enough to measure it. For kinetic measurements, that means quantifying the rate of the CH2OO decay and how that rate changes when other reactants are added. Another difficulty is in distinguishing the Criegee intermediate from similar-looking molecules. For example, the simplest Criegee intermediate, CH2OO, is an isomer of formic acid, HCOOH, which is also an atmospherically important molecule.
Ah-CH2OO
To overcome the first difficulty, the Sandia researchers created their CH2OO not by ozonolysis but by reacting CH2I with O2, as shown in figure 1b. That reaction works well because it produces CH2OO without too much destabilizing vibrational energy and because it doesn’t produce any byproducts that react too quickly with the CH2OO. Since CH2I itself is not a stable molecule, they made it by exciting CH2I2 with a laser pulse, which breaks one of the carbon–iodine bonds and initiates the reaction.
Finding the right reaction to produce CH2OO took a combination of trial, error, and inspiration. The researchers had already tried several less successful reactions when, in the spring of 2011, Arkke Eskola from the University of Helsinki in Finland gave a talk at Sandia about his work on the CH2I + O2 reaction that showed a near-100% yield of I atoms. 2 Taatjes and Osborn wondered what the other product might be. They found that it was CH2OO.
To prove it—to distinguish CH2OO from formic acid and other similar-looking molecules—the researchers used photoionization mass spectrometry. Mass spectrometry sorts a sample of ionized molecules by their mass (really their mass-to-charge ratio). When the ionization source is a beam of tunable, nearly monochromatic vacuum UV photons—available at the Advanced Light Source synchrotron at nearby Lawrence Berkeley National Laboratory—it’s possible to distinguish between molecules with the same mass but different ionization potentials. The ionization potential of CH2OO is 10.0 eV; of formic acid, 11.3 eV.
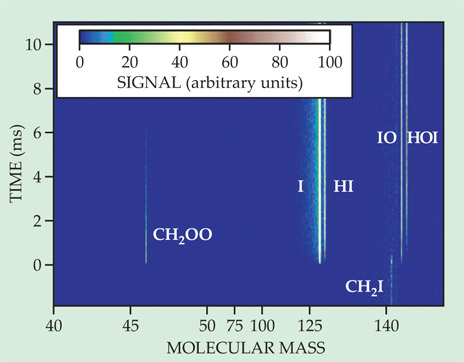
The Sandia researchers used an instrument Osborn had designed a few years before, which continuously records a mass spectrum of a reaction mixture as a function of time. 3 Figure 2 shows the time-resolved spectrum for the CH2I + O2 reaction for a photoionization energy of 10.5 eV. The Criegee intermediate forms rapidly and decays away within a few milliseconds. Adding another reagent, such as SO2, to the mix shortened the CH2OO lifetime. By varying the SO2 concentration, the researchers extracted the reaction’s rate constant.

Figure 2. A time-resolved mass spectrum of the reaction in figure 1b. At time 0, a laser pulse initiates the reaction by dissociating CH2I2 to create CH2I. (The CH2I signal that appears before time 0 comes from CH2I2 molecules that break apart in the ionizer.) The Criegee intermediate CH2OO appears at mass 46. Other primary and secondary reaction products appear at higher masses. (Adapted from ref.
Faster and slower
For three of the four reactions studied, the directly measured rate constants differed by orders of magnitude from the indirect estimates that had been derived from ozonolysis experiments. The reactions with SO2 and NO2 were much faster than expected, and the reaction with NO was much slower—in fact, there was no measurable reaction with NO at all. “Really, it seems like we were surprised by almost every aspect of the results,” remarks Taatjes.
Collaborators Carl Percival (University of Manchester) and Dudley Shallcross (University of Bristol) analyzed some of the atmospheric implications. Among the products of the SO2 and NO2 reactions are SO3 and NO3. A rapid reaction between SO3 and H2O gives sulfuric acid, H2SO4, which contributes to atmospheric aerosol formation; NO3 drives much of the chemistry of the atmosphere at night. Percival and Shallcross estimated that Criegee reactions could produce 40% as much NO3, and more than 100% as much SO3, as other known sources of those molecules.
They had to make some assumptions, though, since so far the Sandia team has studied only the smallest Criegee intermediate at only one temperature and pressure. Future experiments should clarify the picture.
References
1. O. Welz et al., Science 335, 204 (2012). https://doi.org/10.1126/science.1213229
2. A. J. Eskola et al., Phys. Chem. Chem. Phys. 8, 1416 (2006). https://doi.org/10.1039/b516291b
3. D. L. Osborn et al., Rev. Sci. Instrum. 79, 104103 (2008). https://doi.org/10.1063/1.3000004
More about the authors
Johanna L. Miller, jmiller@aip.org




