Xenon chemistry under pressure
DOI: 10.1063/PT.3.3255
Like other noble gas elements, xenon strongly resists forming chemical bonds. Indeed, that inertness makes its isotopes important tracers of the evolution of planetary atmospheres and interiors. Yet the fact that the element can react, albeit reluctantly, was predicted a century ago by Walther Kossel (and later, in 1932, by Linus Pauling), who realized that Xe, with its soft, highly polarizable p shell and relatively small ionization potential, should form compounds with strongly electronegative atoms such as fluorine or oxygen.
That prediction was borne out in 1962 when Neil Bartlett noticed an orange-yellow solid precipitate as soon as he exposed Xe to platinum hexafluoride gas. Syntheses of dozens of Xe compounds soon followed, nearly all containing fluorine or oxygen. But all the xenon oxides produced in the half century since that first demonstration of reactivity have proven unstable at ambient conditions, and some are dangerously explosive.
Applying pressure can forestall the instability. At 100 GPa the change in internal energy of a material may reach several eV per atom. Under such a harsh squeeze, existing chemical bonds can be broken and new ones formed. 1 At 50 GPa an atomic crystal of Xe, which melts at −112 °C at ambient pressure, turns into a compact solid that melts at 3000 °C.
In 2013 Stony Brook University theorists Qiang Zhu, Artem Oganov, and their colleagues predicted that above 80 GPa a sequence of XeOn compounds (with integers n) would become stable against decomposition into atomic constituents. 2 A year later Andreas Hermann and Peter Schwerdtfeger predicted that a host of other candidates, including Xe3O2, should also be stable above roughly the same pressure. 3
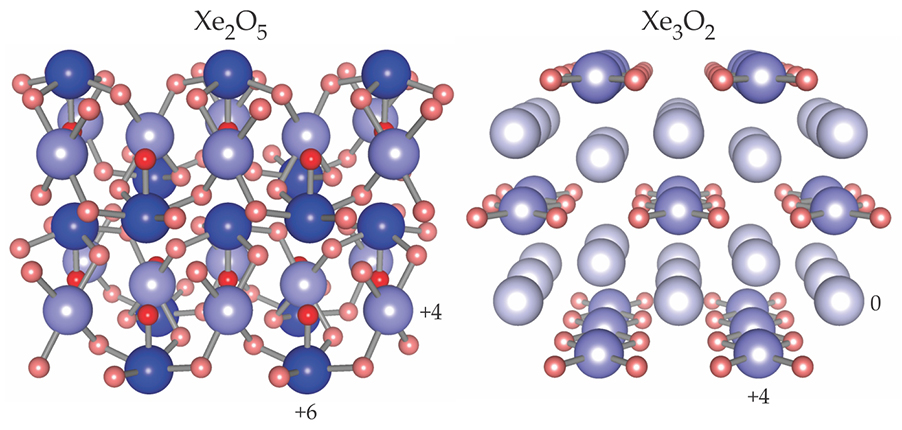
Agnès Dewaele at France’s Atomic Energy Commission (CEA) in Arpajon and her colleagues have now synthesized the predicted Xe3O2 material and a new, unpredicted one, Xe2O5. In their experiments, conducted at the European Synchrotron Radiation Facility (ESRF), oxygen-rich and oxygen-poor mixtures of Xe and O2 were loaded into diamond anvil cells, compressed to near 100 GPa, and heated to 2000 K with an IR laser to induce reactions.
4
The new syntheses, which the group monitored using x-ray diffraction and absorption spectroscopy, represent the first stable xenon oxides made under pressure. The figure on

Xenon oxides, in extremis. When xenon gas is mixed with free oxygen at mantle-like temperatures and pressures, it reacts to form stable compounds of Xe2O5 and Xe3O2. In each structure Xe and O atoms exhibit mixed-valence states. The Xe atoms (blue) are shaded according to their oxidation state (+6, +4, or 0). O atoms are represented by two shades of red, depending on whether they bond with one or two Xe atoms. (Adapted from ref.
Missing xenon
Interest in the xenon oxides is driven partly by a long-standing mystery: Xe’s scarcity in Earth’s atmosphere. At a concentration of less than one part in 20 million, the element is an order of magnitude rarer than expected from the composition of stony, so-called chondritic meteorites. If the gas wasn’t somehow lost to space during Earth’s early, hot accretion, then it must have become sequestered in the planet’s interior. All the near-surface entrapping suspects have been ruled out: Searches for Xe in ices, sediments, shales, and clathrates have turned up far too little to account for what’s missing. The deeper crust and mantle are more appealing as candidate reservoirs because the usually inert element becomes increasingly reactive as pressure and temperature rise.
In 2005 geophysicist Chrystèle Sanloup (now at Pierre and Marie Curie University) and her colleagues squeezed Xe into quartz (SiO2) at thermodynamic conditions on par with those in the crust. 5 They found evidence from x-ray diffraction at ESRF that Xe atoms displace silicon atoms—possibly forming networks of oxide structures, they hypothesized. A few years later, McMaster University chemists David Brock and Gary Schrobilgen strengthened that hypothesis with their ambient-pressure synthesis of XeO2, whose proposed network structure is made up of square-planar XeO4 units, which are thought to replace tetrahedral SiO4 units in quartz. They added crystals of xenon tetrafluoride to water at its freezing point and produced a substance whose vibrational spectra identified it as the sought-for XeO2 (though its crystal structure has not yet been resolved). 6
Dewaele’s new experiment offers a different formation route. It directly exposes Xe to oxygen, the most abundant element in Earth’s lower mantle, at pressures and temperatures within the range of what’s experienced there. Even so, the experiment doesn’t solve the missing Xe problem. For one thing, Xe is so scarce that the element seems unlikely to accumulate in sufficient quantities to be accommodated into deep mineral phases. For another, the O atoms in those deep phases are themselves bound as interstitial components of the minerals, not the free O2 molecules present in the new experiments.
Alternatively, Xe may not form part of a mineral phase at all. Like most other atoms, Xe can be retained at grain boundaries or other defect sites in mantle silicates and oxides. But why the atoms did not then outgas to the atmosphere over geologic time is its own mystery.
Theory to the rescue
The new work is also driven by pure curiosity. The synthesis and identification of new compounds are at the heart of chemistry. Although at first glance the reaction of two gases could hardly be simpler, its interpretation wasn’t. The x-ray beamlines at ESRF are among the most intense in the world, but the diffraction peaks Dewaele and her team observed didn’t yield enough detail for them to reconstruct the number and position of O atoms in the newly produced materials. With its number of electrons less than one-seventh that of Xe, O scatters too few incident photons.
Dewaele also examined absorption spectra of the material to convince herself of the presence of Xe–O bonds and the absence of any bonds between Xe and the carbon in the diamond anvils or the metal in the cell’s gasket.
What’s more, the structures of high-pressure phases can defy chemical intuition. One might suspect that when squeezed tightly together, elements should favor close-packed configurations. After all, atomic Xe sluggishly transforms from one close-packed variant (face-centered cubic) into another (hexagonal-close packed) before it eventually becomes metallic above 130 GPa. But work from the past two decades has proven that supposition wrong. The problem of energy-efficient packing is even more complicated if the spheres are unequal in size.
To resolve the structure and stoichiometry of the new oxides, Dewaele asked Cambridge University’s Richard Needs to predict, using first-principles methods, the most stable, low-energy lattice configurations that should exist under a range of pressures. Reassuringly, the diffraction-peak positions Needs and colleagues Nicholas Worth and Chris Pickard calculated from two of the candidate structures matched the positions visible in the experimental patterns.
To achieve that match, the theorists had to consider Xe’s d-shell electrons as part of the valence shell. The compression essentially opens that otherwise closed core shell thanks to the spatial overlap of the 4d orbitals with the higher-energy 5s and 5p ones. Xe atoms adopt different oxidation states—the number of Xe electrons associated with bonding—in the same compound (see the
More practically, the theoretical treatment of the 4d orbitals produced a more accurate and lower prediction for the pressure at which the new oxides would be stable. As a result, Xe and O2 are more reactive under pressure than theorists had previously realized.
References
1. W. Grochala et al., Angew. Chem. Int. Ed. Engl. 46, 3620 (2007). https://doi.org/10.1002/anie.200602485
2. Q. Zhu et al., Nat. Chem. 5, 61 (2012). https://doi.org/10.1038/nchem.1497
3. A. Hermann, P. Schwerdtfeger, J. Phys. Chem. Lett. 5, 4336 (2014). https://doi.org/10.1021/jz502230b
4. A. Dewaele et al., Nat. Chem. (in press). https://doi.org/10.1038/nchem.2528
5. C. Sanloup et al., Science 310, 1174 (2005). https://doi.org/10.1126/science.1119070
6. D. S. Brock, G. J. Schrobilgen, J. Amer. Chem. Soc. 133, 6265 (2011). https://doi.org/10.1021/ja110618g




