X-ray light valve emerges as a low-cost, digital radiographic imager
DOI: 10.1063/1.3047656
Nearly 15 years ago, University of Toronto’s John Rowlands helped pioneer what has become the state of the art in digital x-ray imaging—the active-matrix flat-panel imager. In the device, a layer of amorphous selenium (a-Se) converts incoming x rays directly to charge carriers that migrate, under the influence of an electric field, into an embedded array of thin-film transistors, amplifiers, and subsequent analog-to-digital converters. The digitized signal can then be displayed, processed, and stored.
The same kind of flat-panel system can also be based on indirect conversion, using both a phosphor layer that emits light when hit by an x ray and an array of photodiodes that convert the light into an electrical signal. But light scattering in the phosphor makes that a lower-resolution approach. (See the article by Rowlands and Safa Kasap in November 1997, page 24
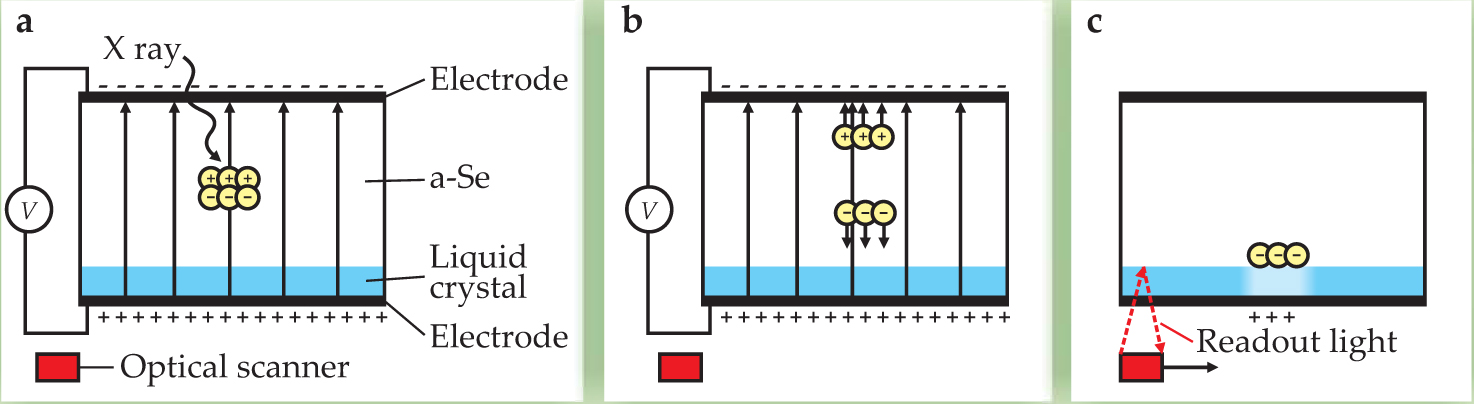
In both cases, image quality is excellent, electronic noise near the quantum limit, and data acquisition fast enough to allow fluoroscopy—real-time monitoring of a changing scene. But because each pixel of the image is individually addressed by its own tiny transistor, active-matrix systems are expensive; a single unit can cost up to $200 000. That puts them out of reach of small hospitals, clinics, and most of the underdeveloped world. Fortunately, Rowlands and colleagues have now developed a device that avoids the expense—the x-ray light valve. 1 , 2 Like an active-matrix system, the XLV relies on a-Se to convert x rays into charge. But unlike that system, the XLV doesn’t measure the charge directly. Instead, it reads the electro-optical effects of the charge through a birefringent liquid crystal. Figure 1 outlines the process.

Figure 1. In essence, the x-ray light valve is a layer of amorphous selenium and thin liquid crystal, sandwiched between two transparent electrodes. (a) An x ray penetrating the a-Se layer creates a cloud of electron–hole pairs via the photoelectric effect. (b) With an electric field applied, some of the pairs drift toward oppositely charged surfaces of the photoconductor where they are trapped. (c) After the applied electric field is removed, the charge distribution from the x-ray exposure induces a visible image in a birefringent liquid crystal by virtue of the crystal’s dielectric anisotropy, which affects the propagation of outside light through it. The optical image is then digitized using a scanner that sends light from an LED through the crystal; the light reflects from the a-Se interface and into the scanner’s array of photodiodes. To reset the light valve for another exposure, the system is flooded with light having energy above the absorption edge of a-Se.
(Adapted from
The cost of the XLV could be an order of magnitude lower than active-matrix systems. “I never saw this as a low-cost system,” says Rowlands, “but one that simply contained some beautiful physics. It took a National Institutes of Health grant for me to realize the cost of a flat-panel imager could be cut without sacrificing image quality.” In November, Rowlands presented the concepts behind his prototype at the Indo-US Workshop on Low-Cost Diagnostic and Therapeutic Technologies held in Hyderabad, India.
An imager’s ingredients
Amorphous selenium is nearly ideal for radiography. It’s exquisitely sensitive to x rays: A single x-ray photon of 50 keV spawns about a thousand electron–hole pairs. The material has a bandgap of about 2 eV, high enough that little dark current flows, and yet low enough to remain a reliable photoconductor. And because it’s amorphous, a-Se can be evaporated as a thick film onto large areas and still retain its optoelectronic properties. The thickness is often tailored to the application: High-energy exposures, such as those used for chest x rays, require a millimeter of a-Se to capture most x rays, whereas a 200-micron layer suffices for the lower-energy exposures of mammography.
Thanks to Chester Carlson, whose work using a-Se made photocopying possible in the 1960s, the literature is rich with accounts of the material’s properties. At room temperature, a-Se is close to its glass transition temperature. So, after the material is evaporated onto a substrate, its defects end up diffusing there and to the free surface in the course of a few days, leaving the bulk largely defect free. Thus, when the material absorbs x rays and a bias voltage is applied across it, the resulting electrons freely drift along field lines until they become trapped at the myriad defect sites at the free surface.
Detecting the presence of those charges is where the liquid-crystal cell comes in. The trapped charge distribution creates a varying electric potential across the cell when it is placed adjacent to the a-Se surface. The liquid crystal acts as a valve. Charge variations induce intensity variations in outside light passing through the cell. The result is a modulated optical image. Moreover, if the latent charge image is not intentionally erased by flooding the a-Se with light above its absorption edge, the image remains for up to tens of minutes, which gives a radiologist plenty of time to capture it in digital form using a separate CCD camera or scanner.
One reason that Rowlands and company hit on liquid crystals is that, unlike the Kerr effect, liquid crystals’ electro-optic effect shows up even when driven by a low voltage. The liquid-crystal layer can also be made as thin as 5 µm or less. The thinner the layer, the smaller the blur introduced during the conversion of a charge image into an optical image.
Indeed, because the XLV interrogates the charge distribution optically, it has the potential to exceed the spatial resolution of active-matrix flat-panel imagers. Like a-Se, liquid crystals are “pixel-less,” discrete only at the molecular level. In active-matrix systems, the pixel size of the detector (typically about 100 µm) limits the spatial resolution. Moreover, the larger the area of the matrix, the greater the distributed capacitance along wires that load the signal amplifier and the greater the electronic noise. The XLV has no array of wires, making its noise properties scale-invariant.
Early on, to extract the optical image, Rowlands coupled a CCD camera to the liquid-crystal screen. But cameras are not efficient light collectors. The inability of the lens to capture all the photons from the screen confers a statistical penalty known as a secondary quantum sink.
The problem prompted him to replace the CCD with an off-the-shelf paper scanner—“a brilliant move forward,” according to J. Anthony Seibert, a medical physicist at the University of California, Davis. Apart from avoiding the secondary quantum sink, the scanner simplifies digitization: A linear array of detectors replaces a two-dimensional matrix of them. The scanner can also be configured to view every part of the liquid crystal from the same angle; it’s thin, which allows it to sit close to the latent image; and it provides remarkably high spatial resolution—the photodiodes resolve up to 1200 dots per inch (21.2 µm), even finer than commercial systems designed for digital mammography. Not least significant, it’s inexpensive. 3
In practice, the research team achieves a dynamic range of just 8 bits from the scanner, although that can be improved by averaging repeated scans. By comparison, medical imagers typically achieve a 10- to 12-bit dynamic range. There’s also plenty of room to optimize the system, says Seibert. Electronic noise from the scanner can be reduced, for example, by increasing the brightness of the LED that illuminates the liquid-crystal image and by improving the scanner’s optics. The liquid crystal itself is also somewhat tunable. A bias voltage across the crystal is required to shift its characteristic curve—the relation between reflectance and applied field—to a region in which the nonlinear crystal’s optical response accurately mimics the spatial variations in the charge image.
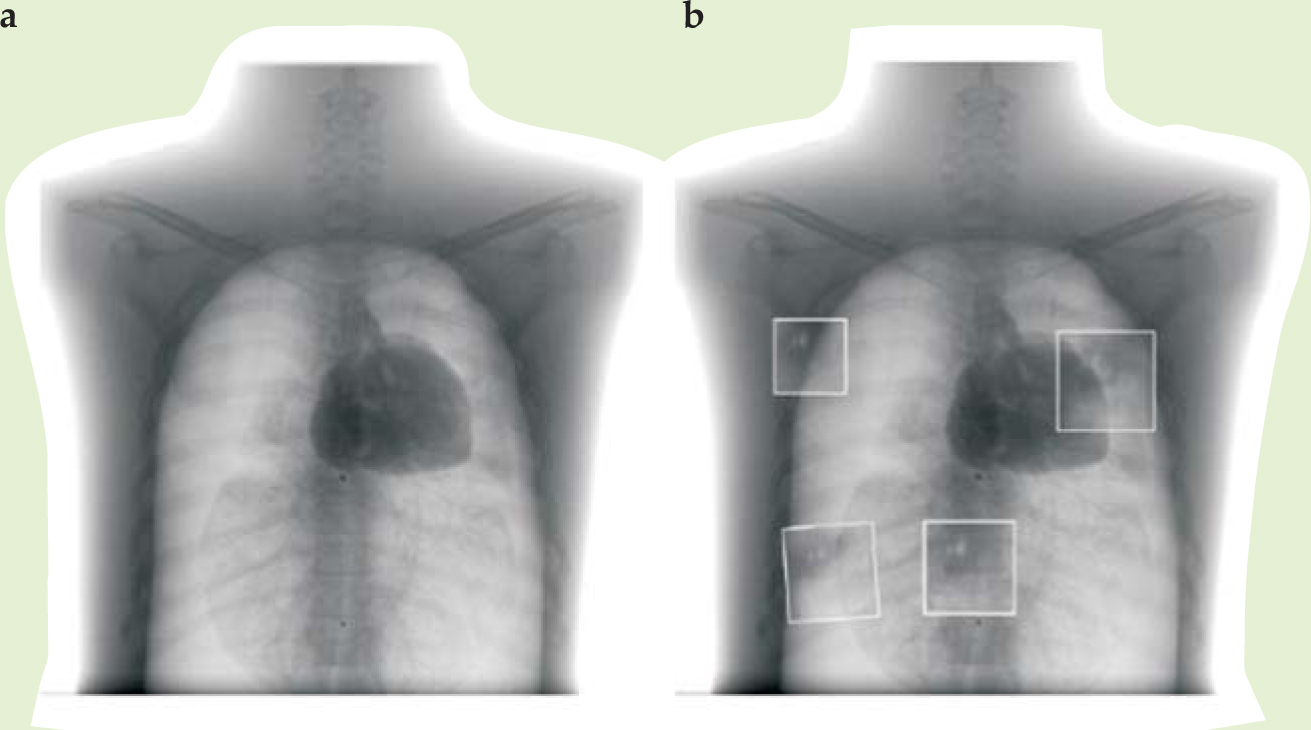
Rowlands envisions the XLV being useful at first for static imaging (in contrast to fluoroscopy) and chest x rays, an application well matched to the system’s dynamic range. He’s now exploring its clinical practicality at Canada’s Thunder Bay Regional Health Sciences Center in northwestern Ontario, where he is setting up a new imaging research institute. Figure 2 compares a standard digital radiograph of a phantom chest using an active-matrix system with some smaller patches using a prototype XLV; “phantom” here refers to a dummy body part that replicates absorption properties of human anatomy.

Figure 2. X-ray images of a phantom chest using (a) an active-matrix flat-panel imager and (b) an x-ray light valve, shown as boxed insets against the AMFPI chest x ray. Imperfections from the prototype x-ray light valve appear as artifacts in the image.
(Courtesy of John Rowlands.)
In developing countries, a crying need exists for simple devices that can ensure bones are set properly and can screen for diseases such as tuberculosis. But Rowlands speculates that reduced cost may affect the technology’s use even in the developed world. In the US alone, hundreds of millions of x-ray exams are performed annually. “It’s somewhat fanciful, but just as PCs and laser printers are now ubiquitous, one can imagine each hospital bed in the intensive care unit outfitted not just with its own heart monitor, but its own x-ray imager.”
References
1. R. D. MacDougall, I. Koprinarov, J. A. Rowlands, Med. Phys. 35, 4216 (2008).https://doi.org/10.1118/1.2968093
2. C. A. Webster, et al., Med. Phys. 35, 939 (2008).https://doi.org/10.1118/1.2837288
3. P. Oakham, R. D. MacDougall, J. A. Rowlands, Med. Phys. (in press).




