The competition is gaining on platinum as a catalyst for hydrogen fuel cells
DOI: 10.1063/1.3141928
Hydrogen is a much discussed alternative to gasoline for powering cars. It can be burned in a fuel cell to produce water and electrical energy. At the anode of a polymer-electrode fuel cell, hydrogen molecules are split into electrons and protons. At the cathode, the protons combine with oxygen and the electrons, which have traveled around an external circuit, supplying power.
A limiting factor in automotive fuel-cell performance is the slowness of the reaction at the cathode. Platinum is effective in promoting that reaction but it is both expensive and scarce. About 75% of the world’s supply comes from South Africa and most of the rest from Russia. To be cost competitive with internal combustion engines, hydrogen fuel-cell cathodes must lower the Pt content by a factor of four.
Platinum’s high price tag has motivated much research on alternatives for the oxygen reduction occurring at the fuel-cell cathode. The focus has been primarily on transition metals such as iron or cobalt that are combined with nitrogen on a carbon support. Last fall, Jeffery Dahn and his colleagues from Canada’s Dalhousie University in Halifax, Nova Scotia, found that Fe–N–C catalysts prepared using an array of methods all performed in a similar manner, with none approaching the desired standard. 1 The researchers concluded, realistically, that it was “time to move beyond” Fe–N–C catalysts. The periodic table is a big place, says Dahn, and needs to be thoroughly explored.
New hope has now been injected into the field by a report from Michel Lefèvre, Eric Proietti, Frédéric Jaouen, and Jean-Pol Dodelet at Canada’s Institut National de la Recherche Scientifique (INRS) in Varennes, Quebec. 2 They fabricated an iron-based catalyst whose activity rivals that of Pt. The current density of a fuel cell made from the new Fe-based catalyst approaches that of a Pt-based fuel cell operating at and above 0.9 V. (The maximum voltage in an ideal hydrogen fuel cell at 80 °C is 1.18 V.)
MIT’s Hubert Gasteiger, who with his colleagues at General Motors in 2005 established a set of benchmark requirements for fuel-cell catalysts, 3 thinks the new result is impressive. He hadn’t believed the high performance requirements for non-precious-metal catalysts could ever be reached. Radoslav Atanasoski of 3M in St. Paul, Minnesota, noted that Dodelet, who heads the INRS team, has been systematically working on Fe–N–C catalysts for more than a decade, and the new work built on that experience.
Dodelet is the first to admit that there’s more work to be done. Two other factors still limit the current performance of the new Fe-based cathodes. One is the insufficient diffusion of oxygen molecules and protons to the catalytic sites. The other is the instability of the catalysts, which degrade with time.
Dahn is also excited about the new results. However, he stands by his group’s conclusion of last fall. The Fe-based catalysts are still a long way from where they need to be, he comments, while Pt catalysts are getting better all the time.
Accumulated wisdom
The exact nature of the active site in Fe–N–C catalysts remains elusive, as does the catalytic mechanism. Years of experiments by Dodelet and others, however, have provided insight into the factors that promote catalytic activity. Unlike Pt-based catalysts, which usually consist of nanosized particles of platinum or its alloys supported directly on larger carbon particles, Fe-based catalysts consist of an iron ion, which is the center of the active catalytic site, linked by ligation through the N atoms to the C support, as seen in figure 1.
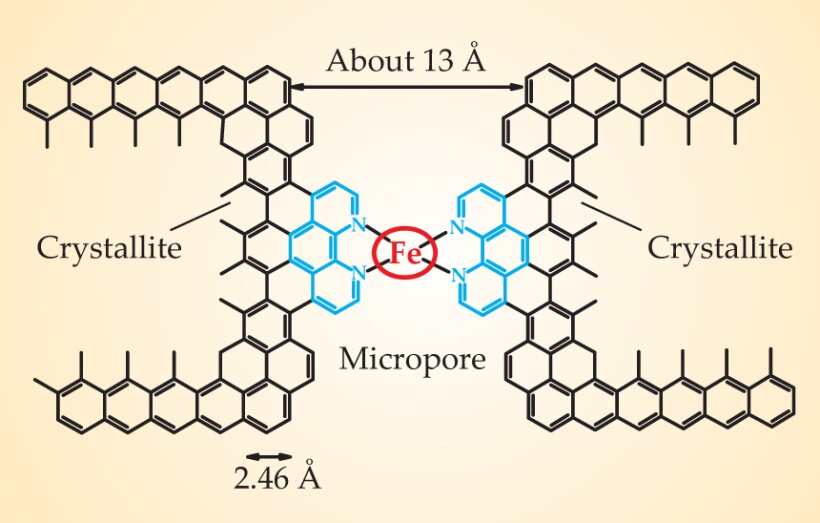
Figure 1. Active site of an iron-based catalyst might look like this. Within a carbon particle are gaps between small graphitic crystallites. Nitrogen can bond to newly formed extensions (blue) at the edge of the crystallites. An iron cation (red) is held by the surrounding N atoms.
(Figure courtesy of Jean-Pol Dodelet, Institut National de la Recherche Scientifique.)
Many researchers today start with chemical precursors containing Fe and N and adsorb them onto some form of C support. They then heat the material to temperatures of 800 °C or more, often in a gaseous environment. The aim is to form attachment sites for the Fe–N complex on the C surfaces.
Dodelet and his INRS coworkers had been heating carbon black with an iron precursor in an atmosphere of ammonia, a nitrogen precursor. A carbon-black particle has small crystallites of carbon separated by regions of disordered C. The experimenters found that the ammonia etched pores in the carbon black, and that those pores with widths less than 2 nm hosted sites where N atoms could bind the Fe ion (see figure 1). They also noted that the etching reaction by ammonia occurred 10 times as fast in disordered C as in structured C crystallites, so the micropores formed primarily in the region of disordered C.
If micropores were so important, the team reasoned, why not simply use commercially available, highly microporous carbon black as the C support? Doing so, however, did not improve the activity of the resulting catalyst. Then the experimenters realized that micro-porous carbon black as delivered was mostly devoid of the disordered C. Hence, the nitrogen essential for ligating Fe ions to the C support could not enter the pores by ammonia etching of disordered carbon.
The experimenters decided to introduce into the microporous carbon black some material that would function as the disordered C. They used a ball-milling technique to force that pore filler, along with Fe acetate, into the micropores. They then heated the resulting material first in argon and then in ammonia.
Resulting performance
Dodelet and his colleagues estimate that the best hydrogen fuel cells produced with their new method extrapolate to a current density of 99 A/cm3 at a fuel-cell voltage of 0.8 V. That is close to the 2010 target of 130 A/cm3 that the US Department of Energy has adopted for oxygen reduction on non-precious-metal catalysts and beats the best previous result by more than a factor of about 30.
Figure 2 compares the performance of a commercially available Pt fuel-cell cathode with that of cathodes made using the best new Fe-based catalysts. The two types perform similarly at low current densities, where performance is governed only by the kinetic activity of the catalyst. However, the voltage for the Fe-based fuel cells falls sharply just above 0.1 A/cm2. Automotive fuel cells would have to operate at around 0.1–1.0 A/cm2, says Jiujun Zhang of the Institute for Fuel Cell Innovation of Canada’s National Research Council.

Figure 2. Performance of catalysts in hydrogen fuel cells. The curves plot the cell voltage, corrected for the Ohmic drop in the cell, against the density of the current on the electrode surfaces, at 80 °C and 1.5 bar. The black curve is a ready-to-use Pt-based cathode with a Pt loading of 400 µg/cm2. The red and blue curves are Fe-based catalysts having total Fe loadings of 90 and 17 µg/cm2, respectively.
(Adapted from
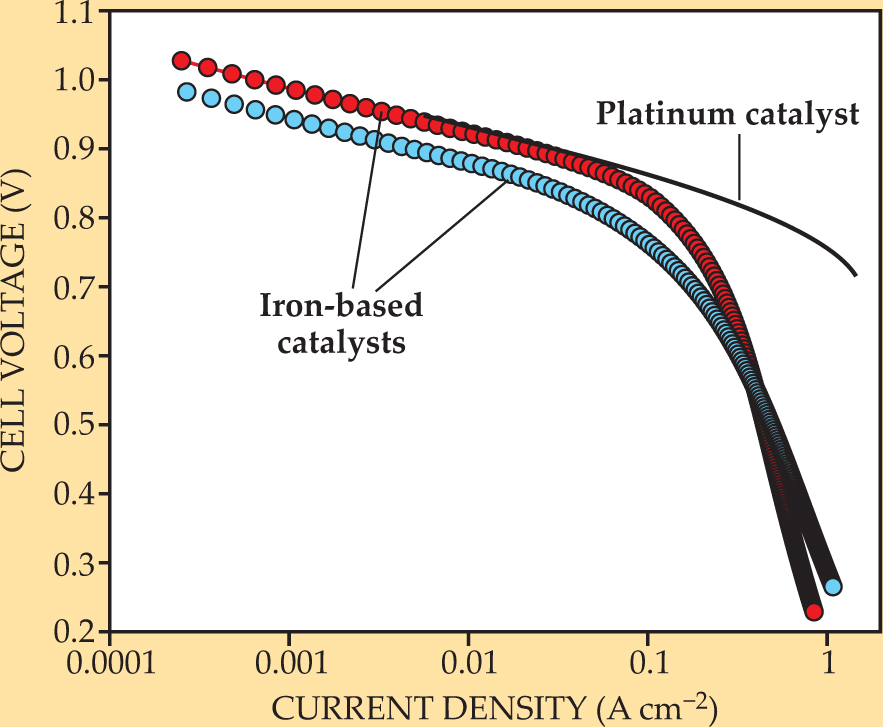
The falloff in voltage is caused by the mass transport problem: To boost the current density for a given voltage, one needs to improve diffusion of oxygen and protons to the active sites within the catalyst. That’s the next hurdle facing scientists and engineers.
Another hurdle is the instability of the Fe-based catalysts, whose cause is currently unknown. Dodelet and coworkers ran their Fe-based fuel cells for about 100 hours, and the current density fell by about 50% within 40 hours. DOE has set 5000 hours as the minimum durability requirement for transportation fuel cells.
A better understanding of the catalytic mechanism, says Zhang, might illuminate the factors that limit activity and help guide the preparation of catalysts and catalyst layers in an optimal and reproducible manner.
Advances in platinum
The Fe-based catalysts may be chasing a moving target. In the past four to five years, experimenters have found ways to increase the activity of Pt above that shown in figure 2. According to Gasteiger and Nenad Marković of Argonne National Laboratory, 4 two ideas will play a role in the next generation of Pt-based catalysts: The first is the “dealloying” of nanoparticles made from alloys of Pt with a transition metal (M), which results in a PtM core and a Pt shell. 5 The second idea is to lay a Pt film merely 10 monolayers thick on a nano-structured support. 6 A less-developed but promising idea is to exploit the finding that the oxygen reduction activity is much greater on some faces of a Pt-nickel single crystal than on others. 7 Researchers propose growing octahedra with those active faces on the surface.
It remains to be seen whether further research on Pt will help offset its cost disadvantage or whether more forward strides will help Fe-based catalysts to overcome their remaining performance deficit.
References
1. A. Garsuch, A. Bonakdarpour, G. Liu, R. Yang, J. R. Dahn in Handbook of Fuel Cells: Fundamentals, Technology and Applications, vol. 5, W. Vielstich, H. A. Gasteiger, H. Yokokawa, eds., Wiley, Hoboken, NJ (2009), chap. 5.
2. M. Lefèvre, E. Proietti, F. Jaouen, J. -P. Dodelet, Science 324, 71 (2009).
3. H. A. Gasteiger, S. S. Kocha, B. Sompalli, F. T. Wagner, Appl. Catal. B 56, 9 (2005). https://doi.org/10.1016/j.apcatb.2004.06.021
4. H. A. Gasteiger, N. M. Marković, Science 324, 48 (2009). https://doi.org/10.1126/science.1172083
5. S. Koh et al., J. Am. Chem. Soc. 129, 12624 (2007). https://doi.org/10.1021/ja0742784
6. M. K. Debe et al., J. Power Sources 161, 1002 (2006). https://doi.org/10.1016/j.jpowsour.2006.05.033
7. V. R. Stamenkovic et al., Science 315, 493 (2007). https://doi.org/10.1126/science.1135941




