The 2008 Nobel Chemistry Prize honors the development of a fluorescent tag for bioscience
DOI: 10.1063/1.3047654
A humble, green-glowing jellyfish has unwittingly revolutionized how researchers study proteins and their activities in living cells. Three researchers whose independent work led to research tools based on the jellyfish’s fluorescent protein have been awarded the 2008 Nobel Prize in Chemistry.
The three equal winners are Osamu Shimomura of the Marine Biological Laboratory (MBL) in Woods Hole, Massachusetts, and Boston University’s Medical School in Boston; Martin Chalfie of Columbia University; and Roger Y. Tsien of the Howard Hughes Medical Institute and the University of California, San Diego (UCSD). The Nobel Prize cites all three “for the discovery and development of the green fluorescent protein, GFP.”
A key feature of GFP is that it does not require the action of an enzyme or other cofactor to turn on its fluorescence. It emits green light in response to stimulation by UV or blue light. Thus researchers can genetically insert GFP to create precisely targeted intracellular genetic tags. The cells then express GFP in conjunction with (or in place of) a protein that researchers want to study. The telltale green glow reports when the gene for a given protein is active. Chalfie likens GFP to a flashlight illuminating the activities in a living cell.
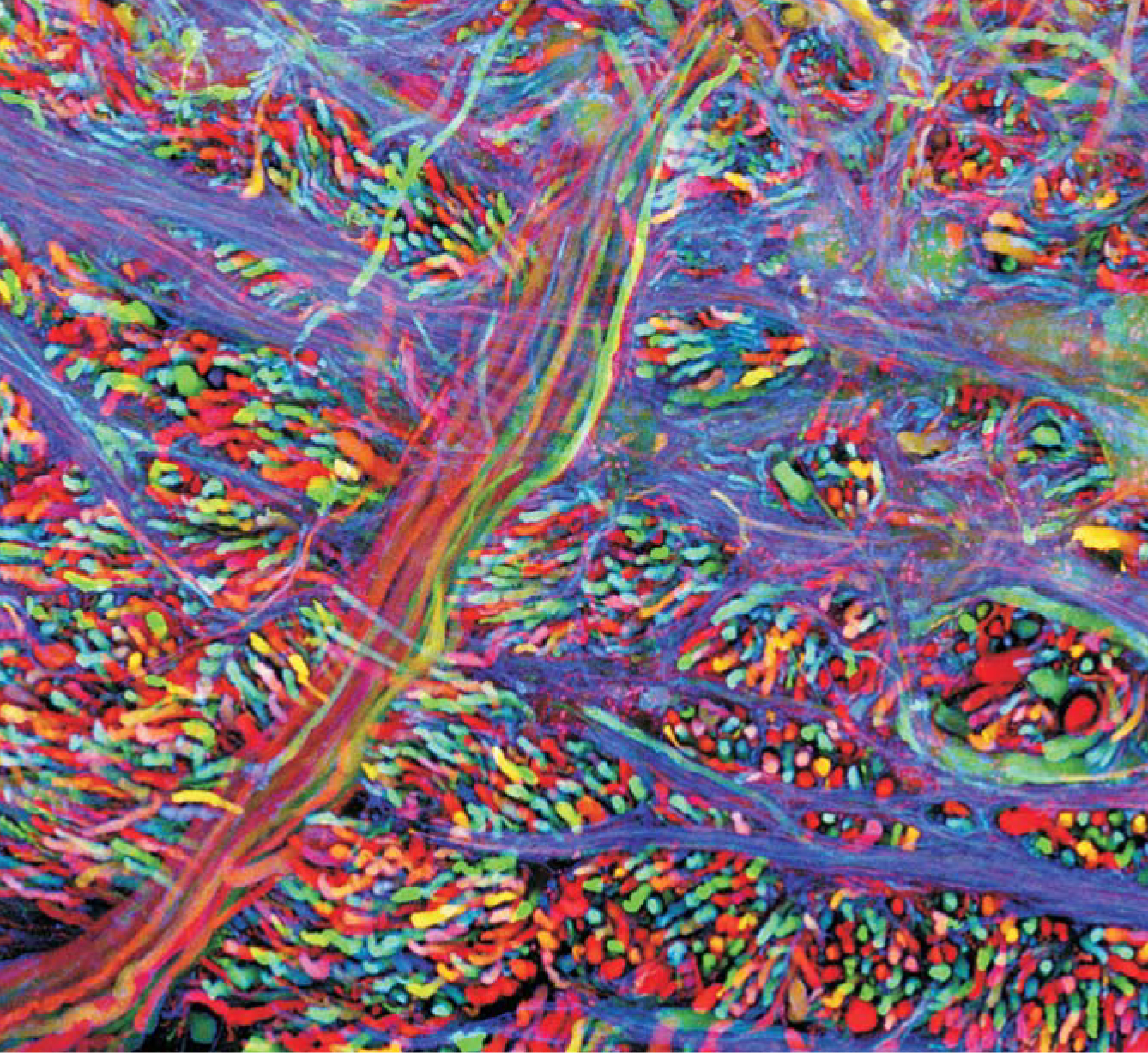
Medical researchers often study the cell’s protein machinery because many illnesses stem from deviations in the machinery’s normal operation. The myriad applications of GFP include studies of how nerve cells develop in the brain, how insulin-producing cells are created in the pancreas of a growing embryo, and how calcium ions flow within the chambers of a beating heart. A particularly colorful application of four differently colored GFP-like proteins is the “brainbow” image 1 of a mouse brainstem seen in the figure on page 22.

A “brainbow” of colors helps researchers see individual neurons in the brainstem of a mouse. By genetic engineering, researchers get each neuron the activate a random mixture of four color genes to produce a total of 90 hues.
(Photo courtesy of Jean Livet and Jeff Lichtman, Harvard University.)
Before the advent of GFP, biologists had to label a protein by inserting into the cell an antibody for that protein and tagging that antibody with a dye. The method was invasive and could not be done in vivo. GFP allows researchers to see proteins in action in a living cell.
Collecting jellyfish
The discovery of GFP in jellyfish began when Shimomura, now 80, was hired by Nagoya University in the late 1950s to extract the substance that caused a bioluminescent mollusk to glow. When Shimomura accomplished the daunting task in only one year, the university granted him a PhD in organic chemistry. Frank Johnson of Princeton University attracted Shimomura to his lab in 1960 to help him study the luminescent jellyfish Aequorea victoria. Soon after his arrival, Shimomura traveled with Johnson and an assistant, Yo Saiga, to Puget Sound in Washington State, where the three labored long hours to cut and filter the bioluminescent edges of nearly 10 000 jellyfish. They then attempted to extract the bioluminescent protein from the remaining “squeezate.”
Shimomura now recalls the frustrating attempts to use conventional techniques to isolate the fluorescent substance. He drifted for long hours in a rowboat to think deeply about the problem. The key to the extraction proved to be the dependence of the jellyfish bioluminescence on the presence of calcium ions found in seawater. In 1962 the researchers reported the discovery of a blue-emitting protein, aequorin, obtained from the jellyfish. 2 Only incidentally did they mention finding a second protein, which did not produce its own light but fluoresced green. That second protein, GFP, turned out to be the important one; it does not require an agent such as calcium to fluoresce.
In the late 1960s and early 1970s, Shimomura and others found that the blue light emitted by aequorin was close to one of GFP’s two absorption lines. The match suggested that the two proteins work as a team in the jellyfish. That is, there is a direct, radiationless transfer of the energy of the chemically excited electric dipole in aequorin to excite the electric dipole of GFP, which emits green light as it returns to the ground state. 3 Experimental work in the mid-1970s by Shimomura and colleagues and, independently, by William Ward and his group at Rutgers University confirmed that such fluorescent resonance-energy transfer was indeed occurring between the two proteins. The process explains why the jellyfish glows green and not blue.
Shimomura also studied the chemical structure of GFP, trying to elucidate the nature of the chromophore responsible for its optical properties. The chromophore structure was not fully resolved until the early 1990s by Ward and his colleagues. 4 In 1982 Shimomura went to MBL and there he pursued other bioluminescent systems.
Cloning the gene
The next step in developing GFP into an indispensable fluorescent tag was to produce the gene for the protein. That task was undertaken by Douglas Prasher as a postdoc in the University of Georgia laboratory of Milton Cormier. Cormier had been isolating and characterizing bioluminescent proteins since the 1950s but in the 1980s he refocused his lab on the cloning of genes involved in bioluminescence. Prasher’s first task was to clone the gene for aequorin, GFP’s partner in the jellyfish. He and his colleagues in Cormier’s lab then turned to the GFP gene. Prasher had moved to the Woods Hole Oceanographic Institution by the time he got the complete gene for GFP in 1992. 5 One key input to getting the gene was the structure of the chromophore as determined by Ward, a former Cormier postdoc.
At the time, no one knew whether GFP required the action of an enzyme or other agent before it could fluoresce. Unfortunately, before Prasher could put the GFP gene into bacteria to see if the host cell spontaneously produced the fluorescent form, his funding ran out. As he now explains, few funders were interested in bioluminescence at that time. Prasher went on to work at different institutions and in other research areas. In 2006 NASA terminated the mission on which he had been working, and today he drives a shuttle van for a Toyota dealership in Huntsville, Alabama.
When Chalfie first heard about GFP at a seminar around 1989, he was immediately excited about its prospects. He thought GFP might be just the thing for looking, literally, into the transparent roundworms (Caenorhabditis elegans) that he had been studying for 15 years. He had always stressed in his lectures that one beauty of the worms was their transparency.
Chalfie immediately contacted Prasher to ask for a copy of the gene, but it was not yet completed. He called again once the gene paper was published in 1992. Prasher shared a copy of the gene with Chalfie and with Tsien, who had also asked for it by then.
According to Chalfie, the DNA coding that Prasher isolated had some extra DNA on it that, in retrospect, would have prevented it from making GFP. Chalfie’s group did not include the extra DNA, leaving only the instructions for making the GFP protein. They inserted the gene into the DNA of Escherichia coli , which then glowed green. The GFP produced by the bacteria spontaneously folded into a fluorescent form, without the aid of any enzymes. The chromophore in the protein is fluorescent only if the protein folds in just the right manner.
Chalfie and his colleagues next used GFP to study roundworms. Chalfie had the idea to insert the GFP gene in the position on the DNA chain normally occupied by the gene for the protein β-tubulin. The worm’s cells then produced GFP everywhere they would normally produce β-tubulin, namely in the worm’s six touch-receptor neurons. Chalfie’s team was able to see where the touch receptors were located and when during development they were turned on. 6 After Chalfie’s paper appeared, the field exploded; more than 20 000 papers involving GFP have been published since 1992.
Not only can researchers replace a given protein with GFP to see where a gene is active, but they can also attach the GFP to a protein to study the protein’s motion and interactions. Such “protein fusion” was demonstrated by a group at Columbia led by Tulle Hazelrigg, 7 Chalfie’s wife. Hazelrigg’s team showed that the protein still functioned normally even with GFP attached to it and that GFP still glowed under excitation. It helps that GFP is a relatively small molecule.
Chalfie, who is 61, received a PhD in physiology from Harvard University in 1977. After spending five years at the Laboratory of Molecular Biology in Cambridge, UK, he went to Columbia in 1982.
Enlarging the palette
When Tsien got his copy of the GFP gene, he and his coworkers at UCSD started studying and modifying the properties of the glowing protein. In 1994 they ascertained that GFP required oxygen, but no other agents, to fluoresce. 8 More strikingly, they found that they could alter the absorption and emission spectra by introducing random mutations into the genes. One of the four fluorescent proteins produced by the mutant genes emitted blue rather than green light. To the researchers’ surprise, that mutation had caused an amino acid to be inserted into the center of the light-producing chromophore.
The robustness of the chromophore to such insertions suggested a way to engineer the optical properties. That method has been widely exploited by many researchers. In 1995 Tsien’s team found a mutation that greatly improved the optical properties of GFP by converting the double-peaked excitation spectrum of the naturally occurring protein to one with a brighter, single peak, which gave brighter fluorescence. 9 Tsien’s group has also introduced mutants that fluoresce in a wide spectrum of colors, including cyan, blue, and yellow.
Completing the spectrum with the color red remained a challenge for GFP-based proteins. One answer came from Sergey Lukyanov and his colleagues at the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry in Moscow. They found a red fluorescent protein called DsRed in an organism closely related to a reef coral. 10 In the same paper, they reported cloning as many as six proteins, including red, yellow, and cyan variants. According to Marc Zimmer of Connecticut College, no one before the Russian team had looked for GFP in corals because the protein does not generate its own light. (They thought GFP would be found only in bioluminescent organisms.)
Tsien found that DsRed was too large to attach easily to proteins because it consisted of four amino acid chains instead of one, as in GFP. He and his colleagues engineered it into a more useful monomeric form that retained its red fluorescence. 11
Another far-reaching technique introduced by Tsien and his colleagues
12
is “circular permutation.” It’s a way to insert entire proteins into GFP without losing the fluorescence. In some cases, light emission is contingent on the interaction of the intruder protein with some other element in the cell’s environment. For example, GFP modified by the insertion of the protein calmodulin glows only when calmodulin binds to certain other proteins, which it does only when Ca2+ ions are present (see May 2006, page 18
Recently Tsien has concentrated on using GFP rather than developing it as a research tool. He is hoping to advance cancer research by directing imaging agents and chemotherapy drugs to tumors. Born in New York in 1952, Tsien got a PhD in physiology in 1977 from Cambridge University. After doing research at Cambridge and at the University of California, Berkeley, he joined UCSD and Howard Hughes Medical Institute in 1989.

Shimomura
Tom Kleindinst


Chalfie
EILEEN BARROSO


Tsien
UNIVERSITY OF CALIFORNIA, SAN DIEGO

References
1. J. Livet, et al., Nature 450, 56 (2007).https://doi.org/10.1038/nature06293
2. O. Shimomura, F. H. Johnson, Y. Saiga, J. Cell. Comp. Physiol. 59, 223 (1962).https://doi.org/10.1002/jcp.1030590302
3. J. G. Morin, J. W. Hastings, J. Cell Physiol. 77, 303–and (1971).
4. C. W. W. Cody, D. C. Prasher, W. M. Westler, F. G. Prendergast, W. W. Ward, Biochemistry 32, 1212 (1993).https://doi.org/10.1021/bi00056a003
5. D. Prasher, V. Eckenrode, W. Ward, F. Prendergast, M. Cormier, Gene 111, 229 (1992).https://doi.org/10.1016/0378-1119(92)90691-H
6. M. Chalfie, Y. Tu, G. Euskirchen, W. W. Ward, D. C. Prasher, Science 263, 802 (1994).https://doi.org/10.1126/science.8303295
7. S. Wang, T. Hazelrigg, Nature 369, 400 (1994).https://doi.org/10.1038/369400a0
8. R. Heim, D. C. Prasher, R. Y. Tsien, Proc. Natl. Acad. Sci. USA 91, 12501 (1994).https://doi.org/10.1073/pnas.91.26.12501
9. R. Heim, A. B. Cubitt, R. Y. Tsien, Nature 373, 663 (1995).https://doi.org/10.1038/373663b0
10. M. V. Matz, A. F. Fradkov, Y. A. Labas, A. P. Savitsky, A. G. Zaraisky, M. L. Markelov, S. A. Lukyanov, Nat. Biotechnol. 17, 969 (1999).https://doi.org/10.1038/13657
11. R. E. Campbell, et al., Proc. Natl. Acad. Sci. USA 99, 7877 (2002).https://doi.org/10.1073/pnas.082243699
12. G. S. Baird, D. A. Zacharias, R. Y. Tsien, Proc. Natl. Acad. Sci. USA 96, 11241 (1999).https://doi.org/10.1073/pnas.96.20.11241




