Stretchy molecules rupture far from the crack
DOI: 10.1063/PT.3.4673
Anyone who’s ever stuck a pin into a balloon knows well what happens next: The tiny puncture swiftly grows into one or more fractures that propagate across the balloon’s surface. Captured by high-speed photography, as shown in figure
Figure 1.

In the blink of an eye, a single pinprick can prompt an inflated balloon (shown here filled with water) to tear itself to shreds: The energy stored in the stretched latex is more than enough to power the rapidly propagating cracks. When less energy is available, damage in an elastic solid develops more slowly. The molecular details of material failure are key to how the process plays out. (Image by Jose Luis Stephens/Shutterstock.com.)

Inflated balloons store so much energy in their stretched latex that they invariably burst when pierced. But elastic material failure isn’t always so certain. Car tires, soft medical implants, and O-ring seals all routinely experience stresses and strains that may or may not be enough to cause them to rupture. The safe use of elastic materials depends on understanding just how much deformation they can withstand before they break. In other words, how much energy does it take to propagate a crack in a stretchy substance?
That complicated problem involves physics on multiple size scales, from the macroscopic bulk down to individual molecules. For decades, models that seek to span those scales have been stymied by a dearth of information. After all, it’s not possible to just zoom in with a microscope to see what the molecules are doing.
Or is it? Costantino Creton of ESPCI Paris, his recently graduated PhD student Juliette Slootman, and their colleagues have now reported an unprecedented look at the molecular-scale damage in a fractured elastic material. 1 Their experiment relies on new molecules, recently developed by coauthor Robert Göstl, that become fluorescent when ripped apart. The researchers find that many more molecular bonds are broken than anticipated—not just on the newly torn edge but tens of microns away.
Rubber theory
The leading model of fracture in elastic materials stems from a 1967 theory by Graham Lake and Alan Thomas, 2 two scientists from the UK-based Natural Rubber Producers’ Research Association. The organization was founded in 1938 to better understand a major cash crop of what were then the British colonies of Southeast Asia. Today it’s wholly owned by the Malaysian Rubber Board and has been renamed the Tun Abdul Razak Research Centre after Malaysia’s second prime minister.
Rubber, as Lake and Thomas knew, is made of a random tangle of polymer chains. What makes it a stretchy solid rather than a goopy liquid are the chemical cross-links that bind the chains together where they touch. To break a solid piece of rubber in two, all the covalent bonds that connect atoms on opposite sides of the fracture plane must be severed.
But simply adding up the dissociation energies of all those bonds gives a value far smaller than the experimentally measured energy required to propagate a crack in the material. Lake and Thomas’s insight explained the discrepancy.
It’s not possible, they reasoned, to tug apart only those bonds that lie in the plane of the crack. Rather, all the bonds in each polymer chain—at least between two successive cross-links—must be subject to the same tension. Only one bond per chain might break, but all the others must be stretched almost to their breaking point, which takes almost as much energy.
The theory works well in some situations, but it fails to explain many observed phenomena, such as the fracture energy’s dependence on temperature and its correlation with crack propagation speed. Researchers thus assume that a certain amount of extra energy is consumed through viscoelastic dissipation—the jostling of polymer chains against one another—in the highly stressed region just ahead of the crack tip (visible in figure
Figure 2.

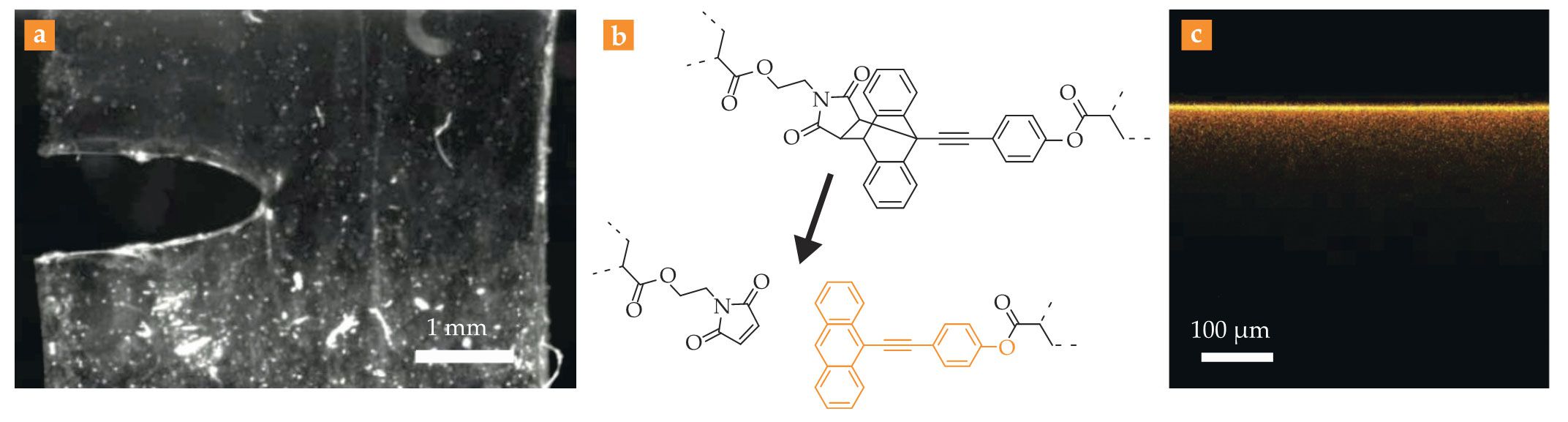
A molecular view of elastic fracture. A notched transparent elastic sheet (a) is put under increasing stress until it ruptures. (b) The sheet has been infused with molecules specially designed to break under tension. The orange portion of the broken molecule is fluorescent. (c) By placing the torn halves of the sheet under a fluorescence microscope, researchers can therefore visualize the spatial extent of molecular damage. Here, the fluorescence signal reveals that chemical bonds many tens of microns away from the nascent crack can still break. (Adapted from ref.
That ad hoc fix isn’t physically satisfying in all cases. Among other things, it seems to imply that molecules jostle one another on length scales even smaller than the intermolecular separation. But for lack of any direct insight into what the molecules were really doing, it was the best that researchers could manage.
Molecular probes
Plenty of experiments have attempted to look at the molecular dynamics of soft materials, often by incorporating specialized molecules that convert the effects of mechanical stress into an optical signal of some kind. In 2014 Creton’s group investigated the properties of a novel elastic material, made of two distinct but intertwined polymer networks, using a molecule that emitted photons immediately when its chemical bonds were severed. 3 The real-time nature of the signal is useful in some cases, but performing optical microscopy at the same time as a mechanical fracture test is cumbersome—and when Creton and colleagues tried using the same molecule to investigate the fracture of a simple, single-network material, they saw no signal at all.
As a postdoc at Eindhoven University of Technology in the Netherlands in the mid 2010s, Göstl wanted to develop a molecule whose mechanical response to stress could be optically investigated after the fact rather than in real time. He had several other desirable properties in mind, including a visible-wavelength signal and the absence of signal from the molecule’s unstressed state. He also sought a high quantum yield, so useful results could be obtained without incorporating so many copies of the molecule that they change the material’s bulk properties. “Previous molecules had one or more of these properties,” he explains, “but they were never all available in the same molecule.”
Figure
Distant damage
Now a group leader at the DWI–Leibniz Institute for Interactive Materials in Aachen, Germany, Göstl collaborates with groups such as Creton’s to use the molecules he developed to solve problems in soft-matter research. Slootman, who trained and worked as a chemist before switching to polymer science for her PhD, had the necessary skills to synthesize elastic sheets of polymethyl acrylate and polyethyl acrylate that incorporated 0.02% of the stress-sensitive molecules. She then ruptured the sheets and looked at them under a fluorescence microscope.
The largest concentration of fluorescence lay on the crack surface itself—the bright yellow line in figure
In molecular terms, that’s enormous. A covalent chemical bond is a bit longer than 0.1 nm, and even the distance between successive cross-links in an elastic network rarely exceeds tens of nanometers. If a single bond were enlarged to the height of a human, 100 µm would be the distance from Chicago to New York City.
As the experiment reveals, not only does molecular damage in a fractured elastic sheet span more of the material than the Lake–Thomas theory accounts for, but there’s also a lot more of it. All the bonds in the fracture plane still have to break, and the distant broken bonds are extraneous to that requirement. A much larger portion of the fracture energy than anticipated therefore goes into severing chemical bonds—and much less energy is left for viscoelastic dissipation.
The result brings the researchers closer to a much-needed physically faithful model of fracture in elastic materials. It offers hope of eventual solutions to multiscale problems, such as how elastic energy stored in the material bulk finds its way to single molecular bonds and how to rationally design new materials that are more resistant to damage.
It also highlights the power of interdisciplinary research. “Creton alone wouldn’t have had the tools to answer his questions,” says Göstl, “and I alone don’t have the polymer-science understanding to pose the questions. Only by closely exchanging ideas can we tackle the big, fundamental challenges.”
References
1. J. Slootman et al., Phys. Rev. X 10, 041045 (2020). https://doi.org/10.1103/PhysRevX.10.041045
2. G. J. Lake, A. G. Thomas, Proc. R. Soc. A 300, 108 (1967). https://doi.org/10.1098/rspa.1967.0160
3. E. Ducrot et al., Science 344, 186 (2014). https://doi.org/10.1126/science.1248494
4. R. Göstl, R. P. Sijbesma, Chem. Sci. 7, 370 (2016). https://doi.org/10.1039/C5SC03297K
More about the authors
Johanna L. Miller, jmiller@aip.org




