Simple compound manifests record-high thermoelectric performance
DOI: 10.1063/PT.3.2404
In 1821 Thomas Seebeck measured a magnetic flux through the plane of a circuit made by joining dissimilar wires and holding the two junctions at different temperatures. Although Seebeck did not recognize it at the time, we now attribute the effect to the migration of electrical charges (electrons or holes) away from the heat source. Materials exhibiting that thermoelectric effect can be characterized by the Seebeck coefficient S, the ratio of the potential drop to the temperature gradient applied.
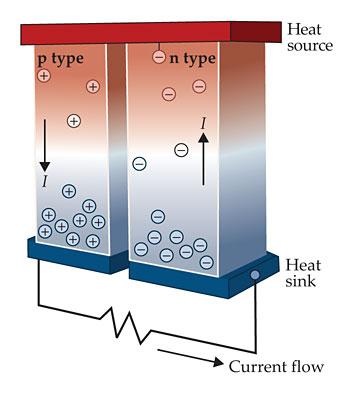
As sketched in figure 1, one can exploit the effect to generate electrical energy from a thermal gradient—or, when run backward, to transfer heat by application of an electrical potential. In the mid 1950s, a brief flurry of research activity was aimed at producing refrigerators with no moving parts. The research on thermoelectrics has accelerated again over the past few decades, 1 driven by a push toward greater energy efficiency: Thermal processes account for 90% of the world’s energy production, so it’s important to squeeze more work out of the exhaust heat.

Figure 1. This thermoelectric device consists of two legs of semiconductor material linking a heat source (red) to a heat sink (blue). Electrons in the n-type leg and holes in the p-type leg move away from the high-temperature end and give rise to a current I flowing through an external circuit. (Adapted from ref.
Studies of thermoelectric materials today are helped by the sophisticated tools available for materials fabrication and characterization. Although practical applications are still not sufficiently efficient to be competitive, “researchers are making good progress,” notes Mildred Dresselhaus of MIT, “and getting close enough to be interesting.” Thermoelectrics see service today primarily in niche markets such as power sources for deep-space probes or coolers for the seats of luxury vehicles, where space is more important than cost. (See the article by Gerald Mahan, Brian Sales, and Jeff Sharp, Physics Today, March 1997, page 42
The efficiency of a device is related to a dimensionless figure of merit, ZT, characterizing each thermoelectric material:
ZT = σS2T/κ,
where σ is the electrical conductivity, S is the Seebeck coefficient, T is the absolute temperature, and κ is the thermal conductivity (the variables σ and κ are temperature dependent). As ZT approaches infinity, efficiencies of heat-engine conversion approach the Carnot limit. Experimenters have been challenged to get ZT much above 1.5. Depending on operating temperatures, ZT = 1.5 corresponds to efficiencies up to 20%.
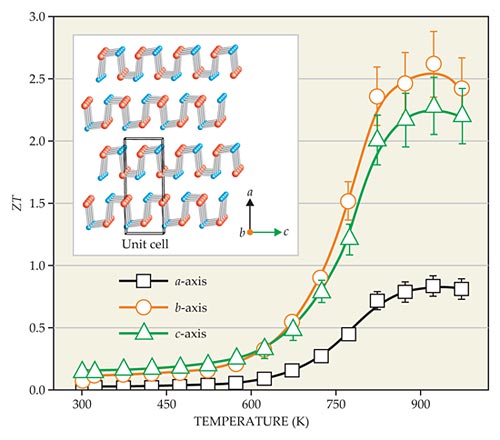
In 2012 researchers achieved ZT = 2.2 in a doped sample of lead telluride. 2 That result has now been topped by a value of ZT = 2.6 measured at high T in single crystals of tin selenide. 3 , 4 The work was done by a team from Northwestern University and the University of Michigan led by Northwestern’s Mercouri Kanatzidis. The measurement of ZT was highest along the b-axis of the orthorhombic crystal, with a slightly smaller value of ZT = 2.3 along the c-axis and a significantly smaller value of 0.8 along its a-axis.
Eric Toberer of the Colorado School of Mines described the surprise of people in the field: “Here is a relatively unexplored material suddenly rocketing to the lead in terms of thermoelectric performance but doing so via very reasonable physics.” The compound had been dismissed as an unpromising thermoelectric back in the 1960s. At the time, researchers had measured its ZT at a low temperature and found it to be unimpressive. The new measurements show that ZT climbs steeply with temperature above 700 K and reaches its peak value around 923 K.
Tin selenide has the additional virtue of being made from elements that might pose fewer concerns for commercial development of products than lead telluride and some other high-performing thermoelectrics. Tin is abundant in Earth’s crust, and selenium is more abundant than the rare element tellurium. And lead’s toxicity is sidestepped.
Ali Shakouri of Purdue University finds the new results intriguing for yet one more reason. “If we can find such significant results in a simple binary crystal,” he asks, “perhaps we still don’t understand the basic tradeoffs. What else might the field have overlooked?”
Electron crystal, phonon glass
The figure of merit ZT is directly proportional to σ and inversely proportional to κ. Hence maximizing its value requires a researcher to find a way to enhance the flow of electrons, as in a metal, but impede that of phonons, as in a glass. That’s a tall order, given that the same factors often favor the flow of both. But it can be done. For example, in one common class of thermoelectric material, based on bismuth telluride, the covalent bonding of atoms within its planes is favorable to electronic currents, but the gaps between those planes hinder the transport of phonons.
Researchers have followed a variety of paths to engineer complex structures with good thermoelectric properties. One way to improve the electrical conductivity is to engineer the energy bands of materials. Another approach is to use nanowires and quantum dots because quantum confinement can enhance the asymmetry of the electronic density of states. 5
To reduce thermal conductivity, teams have designed various alloys in which phonons scatter off point defects, off vacancies, or even off heavy ions rattling around inside cage-like structures. Other teams have fabricated nanostructured materials in which phonons scatter off interfaces. 6
Kanatzidis and his team saw the record-high ZT = 2.6 in a single binary crystal of SnSe. He said they were motivated to look at SnSe because of its unusual structure. It’s a cousin to some common thermoelectrics such as PbTe, which have a simple cubic rock-salt structure. But the SnSe lattice is anisotropic, being a distorted, orthorhombic version of that rock-salt structure.
As seen in figure 2, the crystal features parallel slabs that are each two atoms thick. Within each such slab, some of the bonds between Sn and Se atoms are short and stiff; others (along the a-axis) are long and floppy. The result is a layered structure with weak bonding between the bc-planes. Even within the planes, the atoms are joined in a type of corrugated structure, with soft or floppy bonds mixed in between the stiff ones. The Northwestern–Michigan team suspected the anharmonicity of bonds in the structure would cause weak thermal conductivity in all three directions.

Figure 2. Thermoelectric figure of merit ZT peaks at about 923 K in a single crystal of tin (blue) and selenium (red) atoms. As seen in the inset, the anisotropic structure features two-atom-thick layers within which some bonds are stiff and others weak. The bonds between layers (along the a-axis) are weak. The anharmonic bonds cause ZT to be higher along the b and c crystallographic directions than along the a-axis. (Adapted from ref.
That’s indeed what they saw. To facilitate measurements in the different crystallographic directions, Kanatzidis and his postdoc Li-Dong Zhao grew single crystals of SnSe. They found, as expected, that the thermal conductivity along each direction was quite low—even lower than other state-of-the-art thermoelectrics. Moreover, the values continued to fall even lower as the temperature increased.
The big surprise was that the electrical conductivity and hence ZT were high at elevated temperatures. The researchers had expected those values to be low like the thermal conductivity because of the anharmonicity. Instead, as seen in figure 2, ZT starts low near room temperature but climbs steeply above 750 K to peak around 923 K, even as the thermal conductivity remains about constant over that temperature range. The same behavior was seen in seven different crystalline samples.
Kanatzidis and his team attribute the dramatic increase in thermoelectric behavior to the electronic changes that result from a structural phase transition above 750 K. The high-temperature phase has a reduced energy gap and enhanced carrier mobilities. To test that explanation, the researchers studied the crystalline structure with electron microscopy and calculated the electronic structure. Those studies not only confirmed the key role of the structural phase transition, they also helped the experimenters rule out any impact of possibly inadvertent nanostructures within the crystals.
Practical considerations
The work by the Northwestern–Michigan collaboration is only a start. As with any promising new material, experimenters now must explore its potential for a practical device. One task on their list is to dope SnSe, as is typically done with other semiconductor thermoelectrics, to see what level of doping optimizes the carrier concentration. They must also determine its stability under repeated thermal cycling.
Another property of SnSe that warrants exploration for future application is its highly peaked temperature dependence of ZT compared with the broader peaks seen in most thermoelectrics. In the heat engine shown in figure 1, thermoelectric legs connect the hot and cold reservoirs. To maximize efficiency, it’s best to have a high value of ZT from the hot end, which typically might be 700–900 K, to the cold end, perhaps at 300 K. Further research might address whether the peak of ZT in SnSe can be broadened. Even as is, however, SnSe could still be productively used just for the hot end of the leg, with the rest being constructed from one or more thermoelectrics with high ZT in the lower temperature range.That technique of segmenting is commonly used in cells with large temperature differences.
Shakouri wonders if single crystals might be too expensive for large-scale applications. Most thermoelectrics are usually made from compacting a powder, so one needs to explore whether polycrystalline samples SnSe will retain the same properties as SnSe crystals.
Jeff Snyder and his colleagues at Caltech have been independently working on polycrystalline samples of SnSe. They report that it’s hard to dope the samples and to work with lightly doped material because the charge-carrier concentration is often inhomogeneously distributed. The Caltech measurements of ZT on polycrystalline samples agree with those of Kanatzidis and collaborators up to about 700 K, but the samples were not sufficiently stable to obtain reliable results beyond 750 K. “Given the excitement over the new result on single crystals,” comments Snyder, “further studies are warranted.”
References
1. See, for example, G. J. Snyder, E. S. Toberer, J. Snyder, Nat. Mater. 7, 105 (2008). https://doi.org/10.1038/nmat2090
2. K. Biswas et al., Nature 489, 414 (2012). https://doi.org/10.1038/nature11439
3. L.-D. Zhao, S.-H. Lo, Y. Zhang, H. Sun, G. Tan, C. Uher, C. Wolverton, V. P. Dravid, M. G. Kanatzidis, Nature 508, 373 (2014). https://doi.org/10.1038/nature13184
4. J. P. Heremans, Nature 508, 327 (2014). https://doi.org/10.1038/508327a
5. See, for example, J. P. Heremans, et al., Nat. Nanotechnol. 8, 471 (2013). https://doi.org/10.1038/nnano.2013.129
6. See, for example, J.-F. Li et al., NPG Asia Mater. 2, 152 (2010); https://doi.org/10.1038/asiamat.2010.138
C. J. Vineis et al., Adv. Mater. 22, 3970 (2010).https://doi.org/10.1002/adma.201000839




