Shedding light on chiral substrates
DOI: 10.1063/PT.3.1320
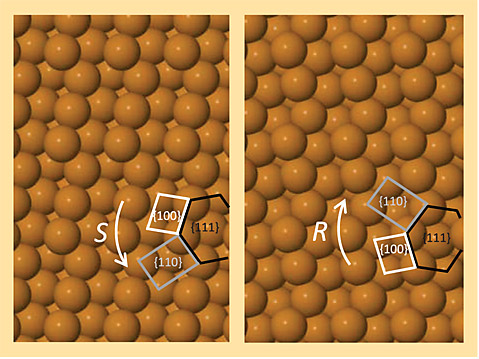
Shedding light on chiral substrates. Most biological molecules naturally occur as either left-handed (S) or right-handed (R) nonidentical mirror images known as chiral enantiomers. Many drugs come in chiral pairs; therefore, pharmaceutical companies are keen to produce chirally pure medicines that bind to their intended target without subjecting the body to the other, unwanted, and in some cases harmful, enantiomer. One novel technique for separating drug mixtures is to pass them over a chiral substrate, typically a metal oxide, that selectively adsorbs specific enantiomers. But in order for researchers to understand the adsorption dynamics, they must first elucidate the substrate’s chiral orientation. Low-energy electron diffraction techniques can be used, but they cannot easily distinguish orientation if the unit cell of the surface is itself achiral. Now, researchers at the Australian Synchrotron, La Trobe University, and the University of Newcastle have shown that chiral orientation can be identified by photoemission techniques. The researchers used 600-eV x rays to excite photoelectrons from a chiral copper substrate, whose S and R orientations are modeled in the images. After measuring the photoemission from multiple angles, they were able to identify the chiral orientation by overlaying the emission distribution, which reflects the substrate’s bulk symmetry, onto geometric lattice projections of the two possible chiral structures. The researchers also showed that chiral orientation can be determined by measuring the photoemission from the Fermi level of substrates that were excited at 200 eV. (