Passive cooling doesn’t cost the planet
DOI: 10.1063/PT.3.3513
Just over 10% of the electricity in the US is generated by the mechanical energy of wind and water. Almost all the rest is produced from heat: from fuel combustion, nuclear fission, geothermal energy, or concentrated sunlight.
The conversion from thermal to electrical energy is inevitably inefficient, so thermal power stations must dispose of a lot of waste heat. Usually that’s done by cooling their turbines and reactors with water. But water is a finite resource that’s not always plentiful around the ideal sites for power plants. An alternative is dry cooling with air instead of water, but those systems are expensive to build, and the large fans that circulate the air consume around 1% of the generated power.
Furthermore, discharging heat into the environment, whether by air or water, means that cooling can’t go below the ambient temperature. Because a thermodynamic cycle’s efficiency is a function of its temperature range, power plants generate significantly less power on hot days—when the demand for electricity is greatest—than they do on cold ones.
Motivated by the challenge of cooling power plants in hot weather, Ronggui Yang, Xiaobo Yin (both of the University of Colorado Boulder), and their colleagues have created a material capable of round-the-clock cooling to below the ambient temperature.
1
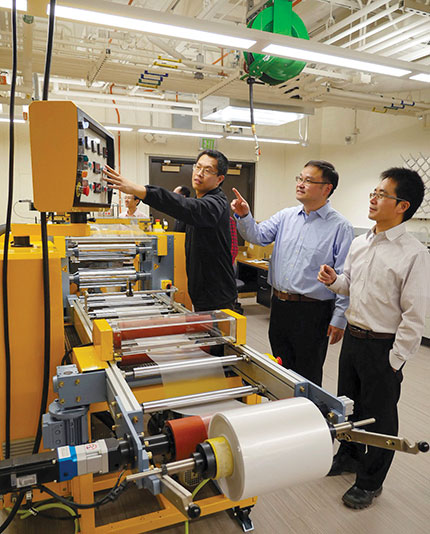
The material, a 50-µm-thick glass–polymer film backed with a 200-nm-thick silver coating, can be cost-effectively manufactured by standard industrial roll-to-roll methods, as shown in figure

Figure 1. Yaoguang Ma, Xiaobo Yin, and Ronggui Yang (left to right) oversee the roll-to-roll production of the glass–polymer film used in their radiative cooling experiments.
GLENN ASAKAWA, UNIVERSITY OF COLORADO
Balance of power
The second law of thermodynamics dictates that heat can’t spontaneously flow from a cooler object to a warmer one, all else being equal. Air conditioners and refrigerators therefore require energy to create and maintain an inside temperature lower than that of the outside air. But the Boulder group’s material, astonishingly, is completely passive. It can cool itself and its surroundings to below the ambient temperature with no power input.
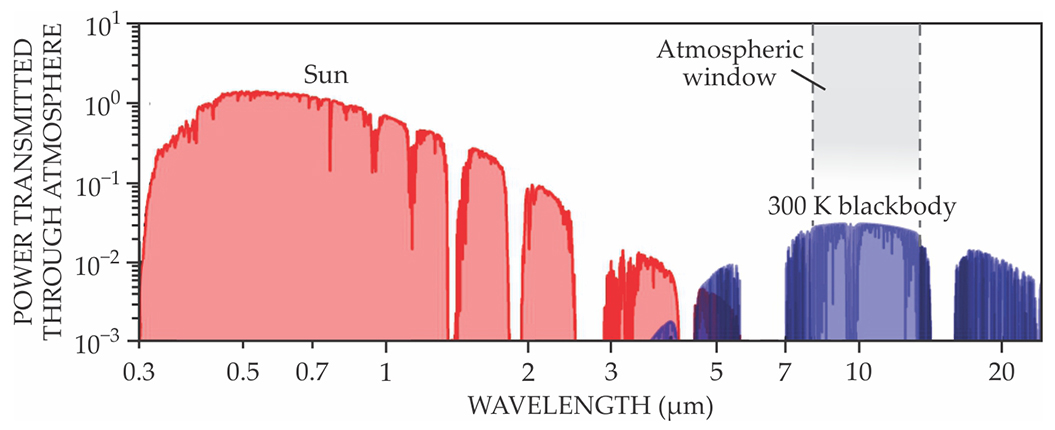
Key to the operation is the atmospheric window: the band of IR wavelengths between 8 and 13 µm over which the atmosphere, greenhouse gases and all, is nearly transparent. As shown in figure

Figure 2. Thermal radiation from the Sun and from a room-temperature object on Earth have little spectral overlap, as shown by these logarithmic plots of the radiative power transmitted through the atmosphere. It’s possible, therefore, to design a material that emits strongly into the 8–13 µm atmospheric-transparency window while being transparent to sunlight. (Adapted from ref.
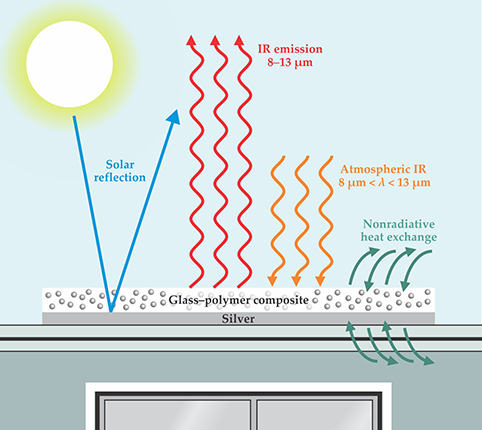
A material’s temperature drops if it can shed more heat than it absorbs. Figure

Figure 3. A glass–polymer cooling panel, depicted here on the roof of a house, must emit more energy into space than it absorbs from other sources: from the Sun, from atmospheric thermal radiation, and from the environment via conductive and convective heat exchange. To keep the sunlight from warming the underlying roof, a film of silver reflects it away.
The mechanism of passive radiative cooling has been understood for a long time, and demonstrations of cooling to below the ambient temperature 2 date back to the 1970s. But nearly all of those experiments were performed at night, when solar heating is not an issue. That made the proof of principle much easier, but in practice, the demand for cooling is greatest during the day. So despite some prototype designs for radiatively cooled rooftop panels, the idea never gained much traction.
Something new under the Sun
Adding sunlight to the mix makes cooling considerably harder. Absorption of just 10% or so of the Sun’s radiation can negate all of a material’s IR cooling power. A daytime cooling material must therefore be almost perfectly transparent to the solar spectrum. In a simple configuration, in which the cooling material is used as a coating to lower the temperature of an object underneath, a layer of reflective metal under the material can reflect away the sunlight. But then the sunlight must pass through the material twice, so it has two chances to be absorbed.
In 2014 Stanford University’s Shanhui Fan and his colleagues demonstrated daytime radiative cooling to 5 K below the ambient temperature. 3 To get the necessary combination of solar reflection and IR emission, they used a nanophotonic material with alternating layers of silicon dioxide and hafnium dioxide. A phonon resonance in SiO2 causes strong IR absorption and emission at around 9 µm, and the layering with HfO2 creates interference effects that broaden the resonance to encompass the entire atmospheric window. To make the layers with precise thicknesses ranging from 13 nm to 688 nm, Fan and company fabricated their material using electron beam evaporation under cleanroom conditions—a process that doesn’t easily lend itself to mass production.
The Boulder group achieved a similar effect with a much less precisely structured material: SiO2 microbeads dispersed in a transparent polymer film. (For their demonstration, the researchers used polymethylpentene, a polymer widely used in medical products. But, they say, any optically transparent polymer should work.) The basis of the IR absorption is the same SiO2 phonon resonance, enhanced and broadened by the beads’ surface modes and collective interactions.
Crucially, no effort was made to control the beads’ exact positions, and their overall arrangement was random—the beads’ diameter (8 µm) and concentration (6% by volume) turned out to generate the required spectral properties by themselves. “We usually think of photonic structures as having to be periodic,” says Yin, “so we were surprised that this random optical structure could work.” That structural freedom allowed the researchers to use scalable production techniques to fabricate their material at a rate of 1.5 m2 per minute, or hundreds of square meters over the course of their experiment.
To measure the material’s cooling power, the researchers connected it to a feedback-controlled electric heater; the rate at which energy was shed into space would match the rate of heating required to keep the system at exactly the ambient temperature. Over three days, they found an average cooling power of 93 W/m2 at midday and well over 100 W/m2 at night.
Inconvenient truths
An air-conditioner replacement that consumes no power is an exciting prospect. “But that’s a long-term goal,” Yang stresses. “We will have to do a lot of thermal system engineering.” Simply placing the cooling material on the roof won’t cool an entire building if the roof is insulated. And most places don’t need constant cooling at night and in the winter, so there would have to be a way to turn the cooling on and off. For example, the material could cool a reservoir of water that’s then circulated through the building as needed. But the details have yet to be worked out.
Another challenge is in limiting or mitigating heat exchange with the environment. As the glass–polymer film drops below ambient temperature, heat flows conductively and convectively in proportion to the temperature difference, and the cooling power drops. In effect, the film cools the atmosphere above in addition to whatever’s below. Because the Boulder researchers used the feedback heater to keep the film at ambient temperature, their measurements don’t account for that efficiency drop. They did do a test without the feedback heater, in which they cooled some water to 8 K below the ambient temperature. But that was at night, without the complication of solar heating.
To best discharge heat into space, the film needs to face a clear, unobstructed patch of sky. Surrounding buildings, trees, clouds, and dirt on the film’s surface could all compromise the cooling efficiency by emitting their own thermal radiation that the film then absorbs. Humidity, too, diminishes the atmosphere’s transparency in the 8–13 µm window and reduces the cooling effectiveness. The Boulder researchers did their tests under nearly ideal conditions: on a series of clear, dry days in an open space in Arizona. “We want to get a better understanding of how atmospheric and geological conditions affect cooling,” says Yang, “but that’s not our area of expertise. And we’re just starting to study the effects of dirt on the film.”
A more immediate application could be adhering the film (without the silver backing) to the surfaces of photovoltaic cells, which can lose efficiency when they get too hot. The researchers expect that the material could be ready for market in as little as a year or two. Beyond radiative cooling, they emphasize the potential to draw on the existing body of research on photonics and spectral engineering to create inexpensive, mass-producible materials. Says Yin, “You don’t need a cleanroom to make a photonic structure.”
References
1. Y. Zhai et al., Science (in press). https://doi.org/10.1126/science.aai7899
2. M. M. Hossain, M. Gu, Adv. Sci. 3, 1500360 (2016) and references therein. https://doi.org/10.1002/advs.201500360
3. A. P. Raman et al., Nature 515, 540 (2014). https://doi.org/10.1038/nature13883
More about the authors
Johanna L. Miller, jmiller@aip.org




