Oscillating chemistry explains complex, self-assembled crystal aggregates
DOI: 10.1063/1.3099567
An exotic class of inorganic crystal aggregates known as silica biomorphs earns its name from a remarkable resemblance to primitive organisms. Indeed, five years ago Juan Manuel García-Ruiz of Spain’s University of Granada, Stephen Hyde of the Australian National University, and their colleagues published a study documenting the near indistinguishability between a variety of biomorph structures and certain Australian bacterial microfossils. 1 A cautionary tale, the paper confirmed that simple inorganic chemistry can give rise to the complex, curved shapes and structures once thought restricted to biological materials. The moral: Scientists who date Earth’s earliest life forms cannot rely on morphology alone to determine what qualifies as biogenic.
Despite its complex shape, a silica biomorph is exceedingly easy to make. The material self assembles when barium chloride (or some other water-soluble alkaline-earth salt) is mixed with a silica-rich solution at ambient pressures and temperatures and at high pH. Carbon dioxide from the air reacts with the barium ions to precipitate barium carbonate. When the carbonate goes on to crystallize in the silica-rich solution, it forms densely packed, nanometer-sized, rodlike particles that are highly ordered in orientation but lack long-range positional order. Those nanoparticles are the building blocks for larger biomorph structures like ones on this month’s cover, but just how they form has long eluded researchers.
García-Ruiz, his Granada postdoc Emilio Melero-García, and Hyde now propose a specific mechanism for the morphogenesis. 2 They argue that the self-assembly boils down to a dynamic interaction of the main components, based on their different solubilities. As carbonate ions come out of solution, the concomitant decrease in local pH triggers the precipitation of amorphous silica. The silica then coats developing carbonate crystals and forms a thin skin around them. And that precipitation of silica, in turn, increases the local pH, which prompts another round of carbonate crystallization in the vicinity. The sensitivity of silica and carbonate species to opposite trends in pH fluctuation creates a chemical feedback that drives the growth process.
Thanks to the oscillating pH at the growth front, the silica-coated carbonate nanoparticles self assemble and aggregate free of the symmetry restrictions normally imposed by the carbonate’s crystal lattice. Perhaps not surprisingly though, the large-scale form of the biomorph depends on the overall pH of the solution, which changes over a few hours due to CO2 dissolution, as well as on local pH fluctuations.
Using video microscopy, the team identifies specific stages of growth. Initially the carbonate crystal grows as a fragmenting, dendritic stalk whose head resembles a cauliflower, as in figure 1. Then, once the pH drops enough for cyclic coprecipitation to be established (within a day, typically), the biomorph grows through pulses of nucleation events to form the carbonate nanoparticles. At that stage, for reasons unexplained by the team’s mechanism, two-dimensional sheets emerge from the 3D cauliflower. Each sheet, consisting of partially aligned nanoparticles, grows radially outward, often to hundreds of square microns, until it curls up at points along its edge like a scroll. Those curls carve out smoothly curved borders that can become shaped like cardiods or interact to spawn filaments like those in figure 2.

Figure 2. Helical filaments emerge from cusps formed when two counterpropagating curling fronts meet each other along the edge of a two-dimensional sheet. The radial velocity v p of the advancing sheet, the azimuthal velocities v φ1 and v φ2 of the curls, and their height H characterize emergent patterns. If the curls have opposite handedness as they approach (top view), the sheet abruptly ends at the cusp. But curls with the same handedness (side view) twist into filaments.
(Adapted from
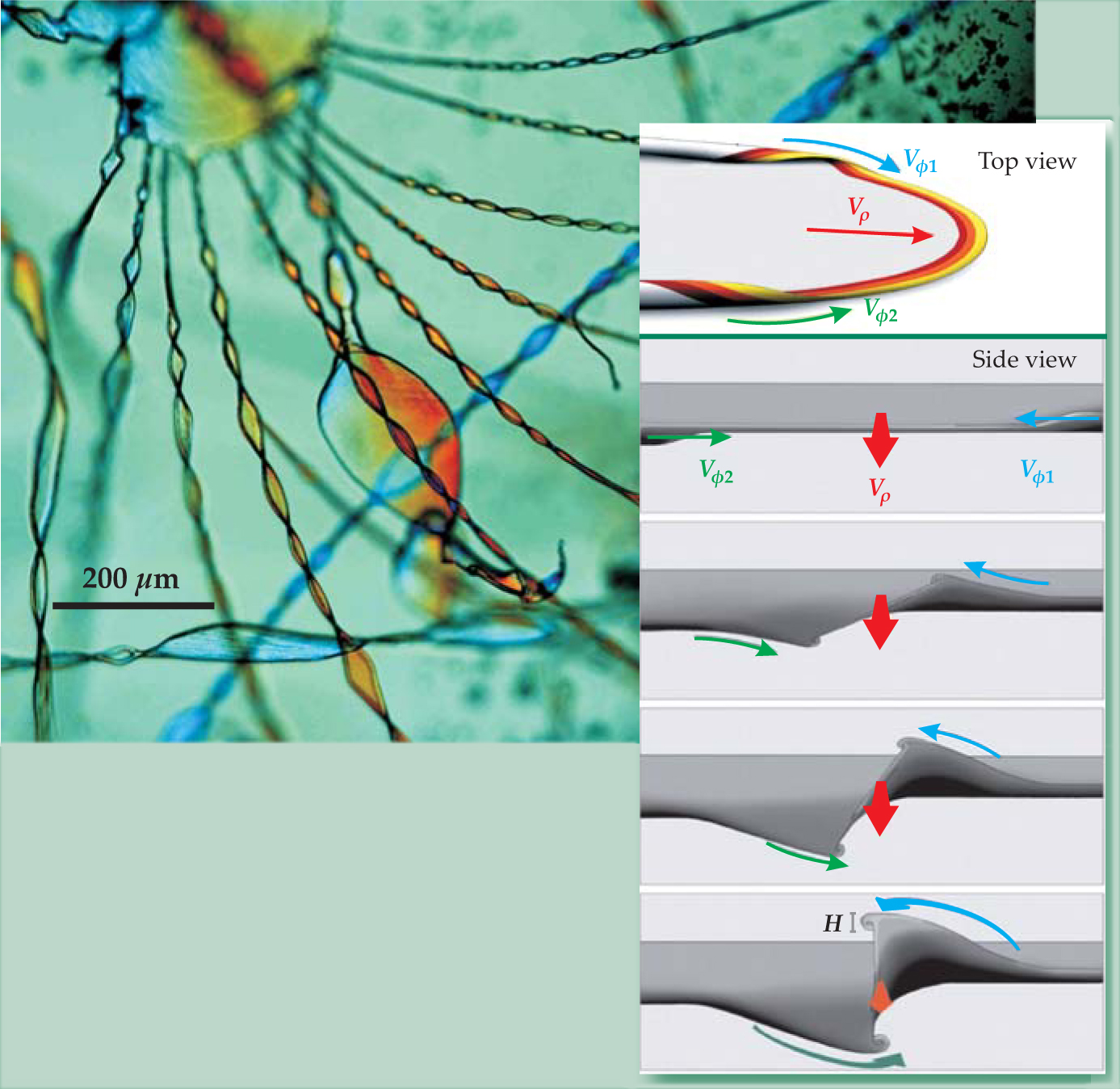

Figure 1. “Cauliflowers.” In highly alkaline solution, carbonate initially grows unchecked as a hexagonal crystalline stalk. But as the pH drops, silica accumulates, randomly adsorbs onto carbonate crystal, and “poisons” its growth; in response, the stalk repeatedly bifurcates into branches. Eventually, enough silica adsorbs onto the cauliflowered ends that carbonate growth effectively ceases, only to be renewed again once pH gradients around silica nucleate additional carbonate crystals and set up the dynamic equilibrium between species.
(Adapted from

Werner Kunz and Matthias Kellermeier (both at the University of Regensburg in Germany) speculate that interfacial tension between the liquid and 2D surface may account for the curling. Michael Marder (University of Texas at Austin) postulates another reason: “Once 2D sheets form, it’s actually hard for them to grow in a way that won’t curl or buckle for exactly the same reason that leaves tend to do so—spontaneous symmetry breaking. Simply inserting material or stretching the sheet changes its internal metric (the equilibrium lengths between things), which induces nonzero Gaussian curvature.” (See the article by Marder, Robert Deegan, and Eran Sharon in Physics Today, February 2007, page 33
García-Ruiz’s team presents a phenomenological model that describes a variety of emergent biomorph shapes using just four parameters: the radial velocity of the advancing sheet; the azimuthal velocity, or speed of the curl’s leading edge; the handedness of the curl; and its relative height. The myriad helical filaments in figure 2, captured by polarized optical microscopy, exemplify one case. Each is extruded from the cusp formed when any two curls moving along the edge of the wide (and out-of-focus) parent sheet approach each other with the same handedness and with nearly equal azimuthal velocities. Together, those conditions lock the curled segments of the sheet into a coupled growth process that causes a filament to twist as it lengthens, as sketched in the side-view inset.
Although mysteries still abound, García-Ruiz argues that the research could provide insight on how biominerals like sea shells, bone, and teeth form. Traditionally, they’re thought to develop from a protein, surfactant, or other biomolecule that, like a scaffold, imprints its structure on the subsequent growth of inorganic minerals. But armed with a plausible mechanism for dynamic self-organization in biomorphs, one can now ask whether that static view of biomineralization is really correct. Whatever the answer, says Kunz, researchers are sure to start searching for novel materials that form out of similar chemically coupled pairs.
References
1. J. M. García-Ruiz, S. T. Hyde, A. M. Carnerup, A. G. Christy, M. J. Van Kranendonk, N. J. Welham, Science 302, 1194 (2003).
2. J. M. García-Ruiz, E. Melero-García, S. T. Hyde, Science 323, 362 (2009).




