Nanoscale electrochemistry
DOI: 10.1063/PT.3.1284
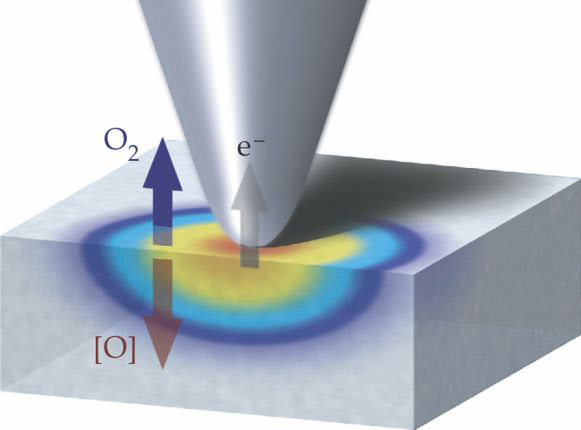
Nanoscale electrochemistry. Although rechargeable batteries and fuel cells have been increasingly considered for or used in mobile electronic devices and electric vehicles, they have not been adopted for large-scale energy-storage and power generation primarily because their energy and power densities are still orders of magnitude below hydrocarbon fuels. Those densities can be greatly improved in metal–air batteries and fuel cells that tap an unlimited supply of environmental oxygen. Studies have shown that the reduction and formation of molecular oxygen in an electrochemical process play a significant role in limiting the efficiencies of those technologies, but insight into the dynamics of those reactions has been hindered by an inability to probe and model them on the nanoscale. Now, researchers from the US, Germany, and Ukraine, led by Sergei Kalinin at Oak Ridge National Laboratory, have employed a scanning probe microscope to map local electrochemical activity on a surface by tracing the appearance, disappearance, and diffusion of oxygen vacancies. As shown in the sketch, a platinum-coated cantilever tip (which doubles as a catalyst) applies a voltage bias to the surface and generates or annihilates oxygen vacancies; the false colors represent the concentration of reduced oxygen. The associated volume change produces an electrochemical strain that is detected by the microscope. Information from the resulting maps may lead to batteries and fuel cells whose mesoscopic architecture is designed to optimize the oxygen-reaction processes. (A. Kumar et al., Nat. Chem. 3, 707, 2011 http://dx.doi.org/10.1038/nchem.1112