Nanodiamonds are promising quantum probes of living cells
DOI: 10.1063/PT.3.1204
Magnetic resonance techniques work by detecting the response of electronic or nuclear spins as they are manipulated by an applied magnetic field. But magnetic resonance imaging and spectroscopy remain notoriously insensitive to faint magnetic moments at small length scales. The performance of induction coils, the standard detectors, scales poorly with decreasing size, and billions of spins are required to yield a recognizable signal. In 2004 Daniel Rugar and his IBM colleagues overcame the problem by combining magnetic resonance with force microscopy. Using a chunk of cobalt at the end of a cantilever, his group was able to locate a single electron spin inside a glass slab (see PHYSICS TODAY, September 2004, page 21
Four years later, two research groups—one led by Mikhail Lukin, Ronald Walsworth, and Amir Yacoby from Harvard University, the other led by Fedor Jelezko and Jörg Wrachtrup from the University of Stuttgart—independently demonstrated an alternative approach that combines magnetic resonance with optical microscopy. 1 , 2 Their method exploits the spin state of a crystal defect in diamond known as an NV center—a nitrogen atom substituted for carbon with an adjacent lattice vacancy. The atomically small defect can, if it’s embedded in a nanoscale diamond, be brought extremely close to a microscopic sample. And thanks to the NV center’s long spin-coherence time, which exceeds a millisecond in nearly pure crystals at room temperature, even tiny magnetic variations on the scale of nanoteslas perceptibly shift the defect’s spin resonance frequency.
When optically excited, an NV center exhibits a stable fluorescence, even in a diamond crystal as small as 5 nm. Because of interactions among its electrons, the defect has magnetically sensitive spin sublevels in its ground state: a spin-zero level ∣0〉 split from nearly degenerate spin-1 levels ∣±1〉 by a microwave transition of 2.9 GHz. That sensitivity allows one to detect weak magnetic fields by observing the spin state, which can be manipulated by microwave pulses and then read out by monitoring the fluorescence intensity. (The intensity depends on which of the spin states is populated.)
Researchers led by the University of Melbourne’s Lloyd Hollenberg have now performed such magnetic-resonance experiments on individual nanodiamonds inside human cancer cells.3 Fluorescent particles have long been used as markers to probe biological processes, but unlike many quantum dots, nanodiamonds are nontoxic and don’t blink, and unlike fluorescent proteins, they don’t bleach under long-term exposure to laser light. That photostability makes nanodiamonds particularly useful for tracking specific organelles, say, or molecular motors in vivo. But Hollenberg wondered if the NV center’s remarkable quantum coherence could be maintained in a cell’s noisy electromagnetic environment. His group’s proof-of-principle experiments demonstrated that it could, albeit with coherence times on the scale of microseconds.
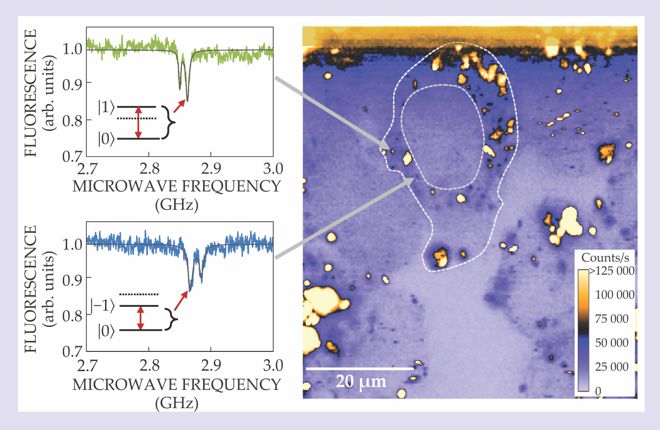
Once the cells ingested the nanodiamonds, the Melbourne team demonstrated how the defects’ spin levels acted both as local magnetometers and as fingerprints to spectrally distinguish each nanodiamond from the others. Although NV centers are chemically stable enough to survive just nanometers from the crystal surface, they experience strain when so confined. Fortuitously, the strain subtly alters each defect’s energy levels and thus its fluorescence spectrum, as shown in the figure. Potentially thousands of NV centers can be uniquely identified and tracked with a precision of 20 nm, and their spin states repeatedly measured for hours.

Point defects known as nitrogen–vacancy (NV) centers in diamond absorb green laser light and fluoresce in the red. In this confocal microscope image (right) of nanodiamonds in living cancer cells, the fluorescence intensity reveals a defect’s spin state, either ms = 0 (brighter) or ms = ±1 (darker). Dashed lines mark the boundaries of a cell and its nucleus. Because each nanodiamond has its own unique size and shape, each NV center experiences a unique strain that breaks the degeneracy of ∣±1〉 spin states and subtly alters the fluorescence spectrum. The spectra (left) thus act as fingerprints to identify, track, and sense the NV centers over hours. (Adapted from ref.
Although it’s possible to anchor a nanodiamond by chemically attaching ligands or proteins that latch onto cellular structures, the researchers allowed their nanodiamonds to freely tumble around the cell. To track that rotational component of the motion, they applied a fixed magnetic field while continuing to measure the spin resonance optically. The resulting orientation-dependent Zeeman shift in energy levels allowed them to deduce the particle’s orientation to within 1°.
To measure an NV center’s rate of decoherence, they used a microwave-pulse sequence to place a defect’s spin in a coherent state—a superposition of ∣0〉 and ∣+1〉, for example—and then made repeated measurements to monitor the characteristic time over which the spin-state oscillations decayed. That work now sets the stage for statistical studies that explore the link between an NV center’s decoherence and local magnetic fluctuations in processes such as the charge transport through ion channels in a cell membrane. Last year Hollenberg and colleagues calculated that ion-channel dynamics could, in principle, be detected with millisecond resolution by monitoring the probe’s decoherence. 4 The issue is not just academic; ion channels are important drug targets.
References
1. J. R. Maze et al., Nature 455, 644 (2008). https://doi.org/10.1038/nature07279
2. G. Balasubramanian et al., Nature 455, 648 (2008). https://doi.org/10.1038/nature07278
3. L. P. McGuinness et al., Nat. Nanotechnol. 6, 358 (2011). https://doi.org/10.1038/nnano.2011.64
4. L. T. Hall et al., Proc. Natl. Acad. Sci. USA 107, 18777 (2010). https://doi.org/10.1073/pnas.1002562107




