Isotope-ratio measurements reveal a young Moon
DOI: 10.1063/1.2883897
Earth is good at concealing its early history. Meteorite bombardment and the convective stirring of surface crust and mantle through the action of plate tectonics have erased the record of what happened during its first 500 million years. Indeed, no actual rocks exist from that era. But current models link Earth’s formation to a series of events in the solar system’s infancy 4567 million years ago.
The date, precisely timed from the decay of long-lived uranium radioisotopes in primitive meteorites, marks the condensation of gas in the solar nebula into fine dust grains. Those dust grains then combined through gentle collisions to form aggregates, planetesimals, and eventually a few tens of Mars-sized “embryos”—all within the first million years of solar-system history. With the heat generated from accretion and the presence of short-lived isotopes such as aluminum-26, those bodies would have melted enough for their dense metals to segregate from the more buoyant silicates. Earth’s later evolution was dominated by the infrequent and traumatic collisions of embryos, a stochastic process that took much longer, perhaps between 10 and 100 million years. 1
Our moon is widely believed to have developed out of the debris ejected by one of those giant impacts with a young Earth. Given the current angular momentum of the Earth–Moon system, dynamical simulations require that the impact delivered a glancing blow. Most of the projectile’s iron core sank into Earth’s, while its silicate mantle vaporized and flew off into orbit. Some 30% of Earth’s mass reached temperatures above 7000 K, and the planet became almost entirely molten, immediately engulfed in a rock-vapor atmosphere. Between a century and a millennium later, the Moon formed
2
(see the article by Robin Canup in Physics Today, April 2004, page 56
When did that event happen? For geophysicists, the question is fundamental. The impact probably added all but the last few percent of Earth’s mass and set the stage for Earth’s and the Moon’s subsequent evolution. To find an answer, researchers turn to rocks returned by Apollo astronauts. The oldest of them, anorthosites found in the lunar highlands, date back 4440 million years, as determined by the samarium-neodymium radiometric chronometer. In addition to Sm–Nd and U–Pb, there are other chronometers, their radioactive parent nuclides all produced by supernova explosions in massive stars just prior to the epoch in which the solar system began.
From measurements of the isotopic composition of tungsten in lunar mantle rock, geochemists Thorsten Kleine (ETH Zürich), his doctoral student Mathieu Touboul, and their colleagues now offer what may be the most robust and accurate constraint on the Moon’s age so far. According to their analysis, the Moon formed at least 60 million years—and perhaps as late as 100 million years—after the birth of the solar system. 3
Measuring the Moon
The hafnium–tungsten system is an ideal chronometer for dating the formation of a planetary core. Radioactive 182Hf decays to 182W with a half-life of 9 million years, and thus dates events that occurred in the first 60 million years or so of solar-system history, before most 182Hf decayed away. What distinguishes the two isotopes is that siderophilic (“iron-loving”) W tends to dissolve in the molten-metal phase that drains downward with gravity, whereas lithophilic (“stone-loving”) Hf remains in the lighter silicate mantle. The segregation leaves the mantle with a high Hf/W ratio; and if the separation occurs before 182Hf has vanished, the mantle will evolve a 182W/184W ratio higher than that of primitive meteorites known as chondrites—objects with the same chemical composition as the solar nebula.
Five years ago a difference in the W isotopic composition between Earth’s mantle and chondrites helped three independent groups of researchers address the time scale during which Earth’s core had formed—roughly 10 million to 30 million years after the birth of the solar system (see Physics Today, January 2003, page 16
For the past decade, researchers have also used the Hf–W chronometer to date the giant, Moon-forming impact. They reasoned that if the different lunar rocks available to them revealed different 182W/184W ratios, then the impact occurred while Hf was still actively decaying. That analysis constrained the Moon’s formation to around 30 million to 50 million years after the solar system’s. Repeated measurements of lunar samples appeared to bear out the isotope-ratio variations.
The timing agreed with theories calling for a rapid accretion of material that formed Earth and a rapid cooling and crystallization of a magma ocean on the Moon and Earth. But it also constrained the Moon’s formation to a narrow window of time and contradicted other radiometric clocks, such as Sm–Nd. The decay of 147Sm to 143Nd, for instance, dates the formation of the first lunar crust that crystallized and floated on the magma ocean around 120 million years after the solar system began. 4 Moreover, the Moon is highly depleted in Fe, which suggests that at the time of the impact, most of Earth’s iron had already partitioned into the planet’s core.
Accounting for the contribution of cosmogenic W to the measured W-isotope variation turned out to be a more significant problem. Cosmic rays incident on the lunar surface release thermal neutrons that are captured by tantalum-181. The 181 Ta becomes unstable 182 Ta, which then decays to 182W. The excess 182W skews the age interpretation in the early direction.
Fortunately, Ta is lithophilic, preferring to dissolve in a silicate matrix. So, to prevent it from interfering with their new analysis, Touboul and colleagues restricted their measurements to lunar metals. Metal grains occur in almost all lunar rocks. The team began with crystallized melt derived from rock deep in the lunar mantle, and separated out the magnetic components in each. Purifying the samples required exquisite care, and the team had to manually pick out magnetic oxides from the metal grains to remove all vestiges of Ta-derived 182W. (Other researchers, including Kleine, had years earlier attempted to remove all traces of Ta, but they had been stifled by a relative scarcity of material and the overlooked presence of the oxide impurities.) Even starting with milligrams of lunar metal—some five times as much material as used in prior studies—the Swiss group performed their mass-spectroscopic analysis on just nanograms of 182W.
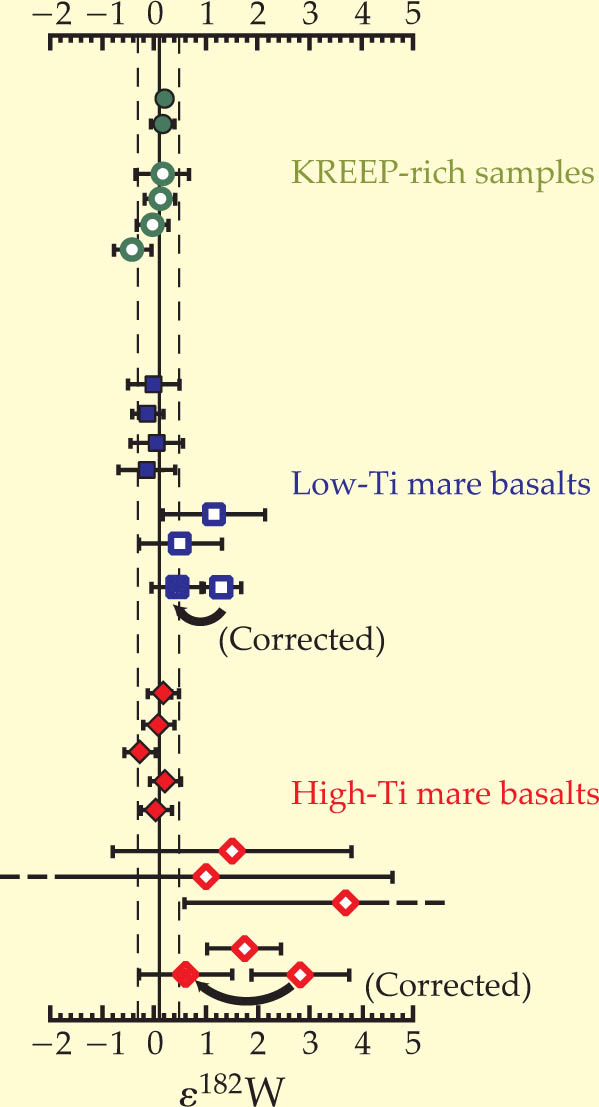
Remarkably, the analysis revealed no variation in the 182W/184W isotopic abundance ratios between Earth’s mantle and the Moon (see figure 1). The Moon therefore formed after 182Hf had vanished. Because the basalts had come from mantle rock in different reservoirs and thus contained different Hf/W chemical ratios, the team could plot what’s known as an isochron. The plot firmly constrains the differentiation of the lunar magma ocean to have occurred no earlier than 60 million years after the solar system began.

Figure 1. Differences in the isotopic abundance of tungsten-182. The parameter ∊ signifies the deviation in parts per 10 000 of the ratios of 182W/184W found in three different types of lunar basalt—melt liquid that rose to the surface by volcanism and crystallized—from the value measured in Earth’s mantle. Recent measurements (filled symbols) provide new data points
(Adapted from ref. 3.)
The W-isotope ratios offer convincing evidence that the Moon inherited its chemical properties from Earth. Indeed, the mantles of the two bodies are isotopically indistinguishable, at least in those regions for which we have samples. That would be no surprise if the bulk of the Moon came from silicate Earth. The paradox, however, is that hydrodynamic simulations of the giant impact require that the vast majority—as much as 80%—of what became lunar material must have come from the Mars-sized impactor that struck Earth.
Could the impactor have had the same mix of primordial material as Earth and thus have evolved the same bulk isotopic composition? That’s hard to imagine unless they grew at exactly the same distance from the Sun, fed from the same part of the solar nebula, and had similar histories of core–mantle separation.
Making the Moon
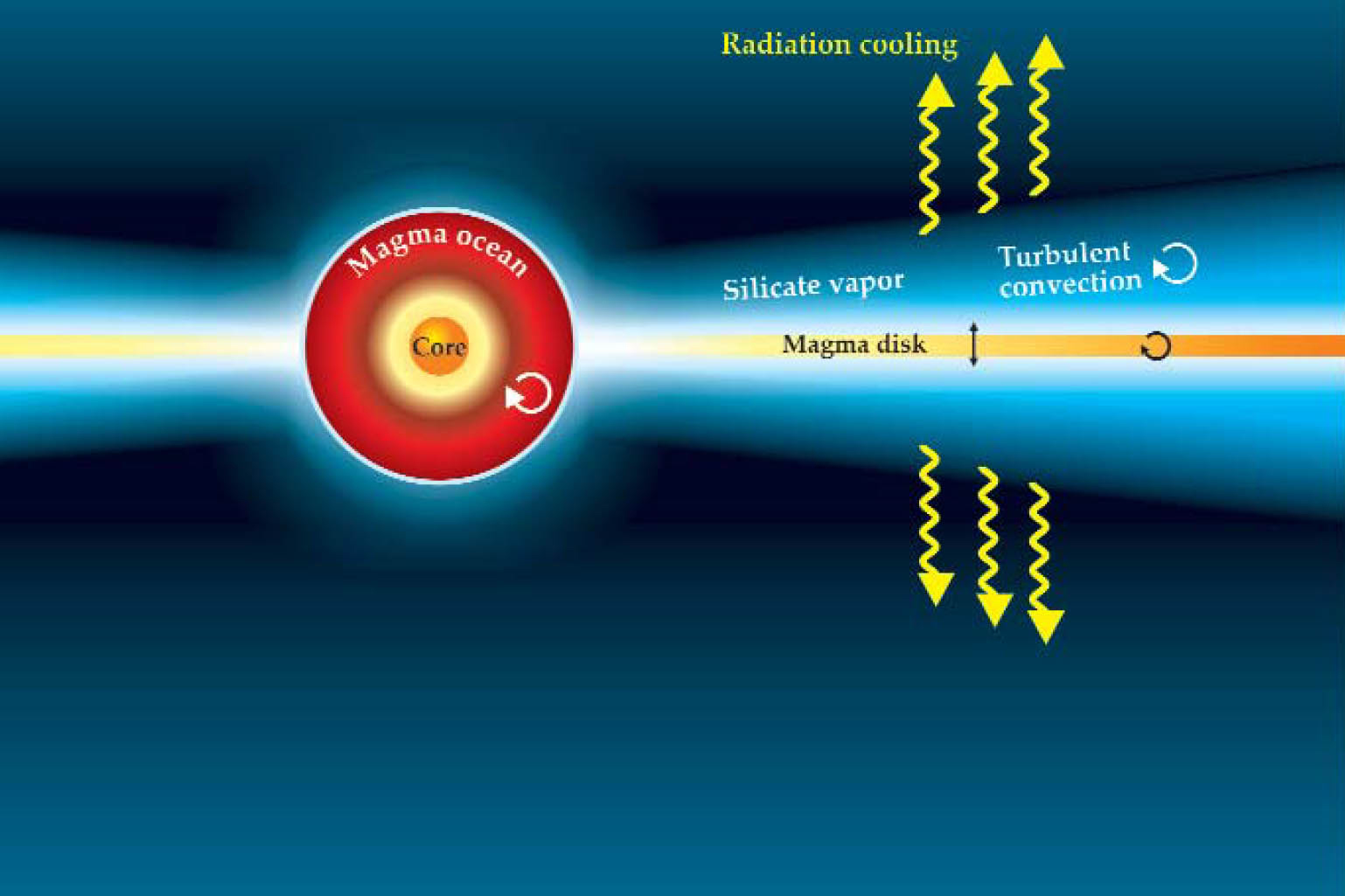
Last year Caltech’s David Stevenson and his student Kaveh Pahlevan addressed the paradox in calculations that show it’s possible to create a moon with Earth-like composition even by starting with an embryo whose isotopic content is dissimilar to Earth’s (see figure 2). During the roughly thousand years required for the orbiting disk of magma and gas to cool and condense, it mixes and exchanges material with Earth through a silicate atmosphere they both share. By virtue of its much greater mass in the collision, Earth can so dilute the smaller object’s individual chemical makeup through turbulent mixing as to virtually eliminate it. 2

Figure 2. Immediately after the giant impact, the Earth–Moon system was largely molten and partially vaporized. A view of the system’s evolution a mere 24 hours later, this schematic shows Earth surrounded by a disk of magma and gas derived largely from the Mars-sized object that struck it. The magma disk and Earth share a rock-vapor atmosphere. According to Kaveh Pahlevan and David Stevenson, turbulent exchange of liquid and vapor allows the two reservoirs to equilibrate on the time scale of 100 to 1000 years.
(Adapted from ref. 2.)
Although the calculations specifically address a previously discovered similarity in oxygen isotopic composition of Earth and Moon, and its difference to all other rocky objects in the solar system, geochemists naturally wonder whether the theory, by extention, can account for the recent 182W data. Stevenson concedes that he’s unsure. Tungsten is extremely refractory, which makes it puzzling that W would mix so efficiently. “One problem is that we don’t really know the vapor compositions of materials coexisting with rock at 2000 K, and there’s the whole periodic table to worry about,” he says.
Moreover, the chemical differences between Earth and the Moon—the total Hf to total W ratio, for example—are not yet understood. And although Earth and the Moon share similar compositions of silicon, magnesium, potassium, and oxygen, the Moon is far more depleted in volatile elements like the alkali metals. Those were probably well mixed in the silicate atmosphere but may have escaped from the lower gravitational field of a hot Moon as it was accreting.
The challenge now is to account for chemical heterogeneities with models—in effect, to build a realistic Moon.
References
1. J. Chambers, Earth Planet. Sci. Lett. 223, 241 (2004).https://doi.org/EPSLA2
10.1016/j.epsl.2004.04.031 2. K. Pahlevan, D. J. Stevenson, Earth Planet. Sci. Lett. 262, 438 (2007).https://doi.org/EPSLA2
10.1016/j.epsl.2007.07.055 3. M. Touboul, T. Kleine, B. Bourdon, H. Palme, R. Wieler, Nature 450, 1206 (2007).https://doi.org/NATUAS
10.1038/nature06428 4. R. W. Carlson, G. W. Lugmair, Earth Planet. Sci. Lett. 90, 119 (1988).https://doi.org/EPSLA2
10.1016/0012-821X(88)90095-7 5. T. Kleine et al., Science 310, 1671 (2005);https://doi.org/SCIEAS
10.1126/science.1118842
D.-C. Lee et al., Earth Planet. Sci. Lett. 198, 267 (2002).https://doi.org/EPSLA210.1016/S0012-821X(02)00533-2




