Ion channels facilitate long-distance communication among bacteria
DOI: 10.1063/PT.3.3041
The membranes of all living cells contain proteins called ion channels that allow ions to move in and out of the cells. The flow of ions through those channels helps to regulate the electrostatic potential difference across a cell’s membrane.
In the case of multicellular organisms, cells communicate with each other by opening and closing specialized channels that let only specific ions through. For example, the coordinated opening and closing of sodium and potassium channels and the associated inflow of Na+ and outflow of K+ propagate a positive voltage pulse—a nerve impulse—down a neuron.
Single-cell organisms such as bacteria also contain various ion-specific channels. In fact, biologists have gained much of what they know about the structure and function of ion channels by looking at proteins harvested from bacteria. What the bacteria use them for has long been a puzzle.
Gürol Süel (University of California, San Diego) and his colleagues have shown for the first time that bacteria also communicate via ion channels. The researchers observed that in densely packed colonies of Bacillus subtilis, the bacteria’s growth cyclically slowed down and sped up. Those oscillations, they discovered, were coordinated by the release of K+ by cells in the colony’s interior. 1
Mimicking nature
Süel and his colleagues suspected that the difficulty in pinpointing the function of bacterial ion channels had to do with the way bacteria are studied. Lab studies typically use bacterial cells suspended in a liquid. “Well-mixed liquid cultures are not how bacteria exist in nature,” he says. Bacteria more commonly form dense colonies of cells called biofilms that attach to a surface.
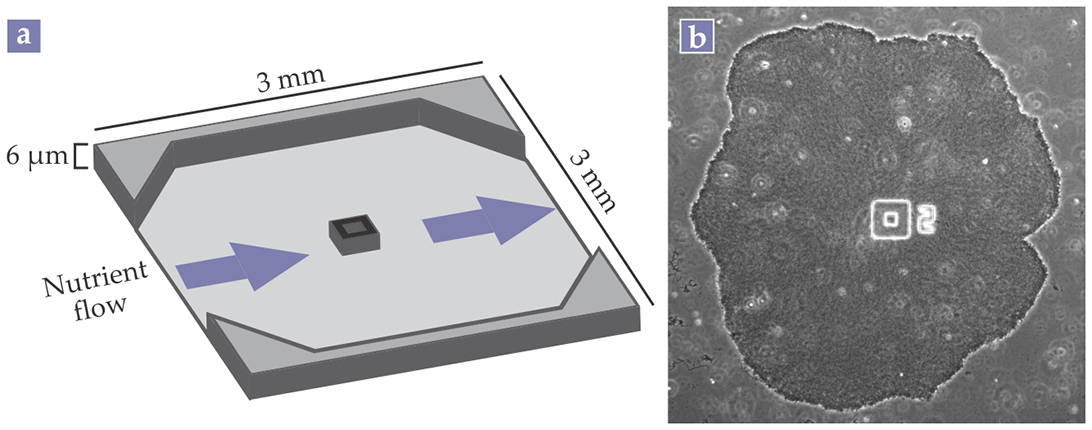
As shown in figure 1, the researchers grew their B. subtilis biofilms in microfluidic devices with continuous flows of a nutrient broth. Süel explains that the microfluidic devices allowed his team to precisely manipulate the environmental conditions around the bacteria.

Figure 1. Bacteria commonly form biofilms, densely packed cellular communities that can contain billions of cells. (a) A microfluidic device with a constant flow of nutrients allows precise control over the biofilm’s environment. (b) The microscope image shows a Bacillus subtilis biofilm grown in such a microfluidic device. The number 2 is a label. The square in the middle of the device is a cell trap that initially helps to attach the biofilm to the surface. (Panel a adapted from ref.
The biofilms exhibited collective behavior that surprised Süel and his colleagues. Despite the continuous flow of nutrients, once a biofilm grew to be bigger than about 600 μm in diameter, biofilm growth began to periodically slow down and speed up. 2 The researchers surmised that those oscillations reflected an internal conflict within the colony between long-term survival and short-term growth.
Cells at the film’s edges protect the interior cells from external crises, such as a sudden change in pH or the presence of antibiotics. The long-term maintenance of interior cells is a critical hedge against such threats. But for the colony to expand, cells at the periphery, where film growth largely occurs, often use up a lion’s share of available nutrients. That overconsumption of nutrients—glutamate in the case of B. subtilis—at the edges can starve the interior.
Süel and his colleagues realized that the biofilm colony balanced those two requirements by developing a metabolic codependence between the interior and peripheral cells. The cells break down some of the glutamate in the nutrient broth into ammonium. They then combine the ammonium with glutamate to make the amino acid glutamine, a necessary ingredient for bacterial growth.
When the interior cells are starved of glutamate, they stop producing ammonium. The reduction in available ammonium is enough to halt the growth of the peripheral cells; glutamate then becomes available in the interior.
Sending out an SOS
Although the ammonium–glutamate balancing act is sufficient to explain the growth oscillations, the high degree of synchronization in those oscillations suggested that additional coordinating mechanisms must be at play. Because a cell’s ability to absorb glutamate or retain ammonium depends on its membrane potential, Süel and his colleagues suspected that the mechanism was electrochemical.
Using a voltage-sensitive fluorescent dye, the researchers found periodic fluctuations in membrane potentials that went hand in hand with the growth oscillations. When they added glutamine directly to the nutrient broth so the bacteria didn’t have to make it out of glutamate, the fluctuations stopped. Those results demonstrated a connection between the growth oscillations and membrane potentials.
The next step was to figure out what ions were involved in the membrane- potential changes. Süel and his colleagues used fluorescent dyes that bind to either Na+ or K+. They found that changes in the extracellular concentration of K+ were directly correlated with the changes in the membrane potential, as shown in figure 2.
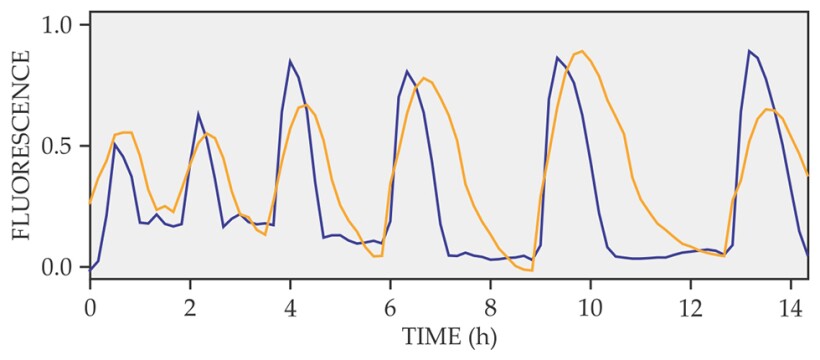
Figure 2. Fluorescence signals from a voltage-sensitive dye (blue) and a potassium-sensitive dye (yellow). Fluorescence is given in arbitrary units. The correlation between the two signals indicates the strong connection between the bacteria’s cell-membrane potential and the K+ release. (Adapted from ref.
They deduced that when peripheral cells overconsume glutamate, the starved interior cells open a potassium-specific ion channel called YugO. As shown in figure 3, the K+ signal propagates across the length of the film. Crucially, the signal doesn’t decay—a sign that neighboring cells actively amplify the K+ signal by releasing their own K+. In effect, the starving interior cells send out a metabolic SOS, and neighboring cells relay that distress call to the biofilm’s periphery like a bacterial bucket brigade.
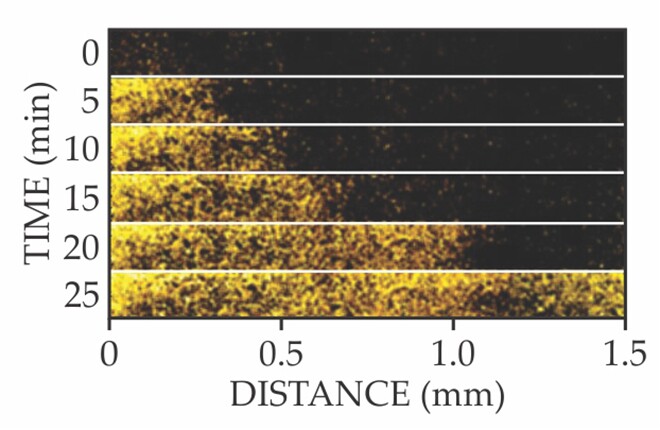
Figure 3. Active propagation of potassium ions. This time series of fluorescence microscope images shows that cells in the interior of a biofilm release K+ (yellow) as a metabolic distress call to cells at the periphery. Neighboring cells actively amplify and relay the signal by releasing their own K+. (Adapted from ref.
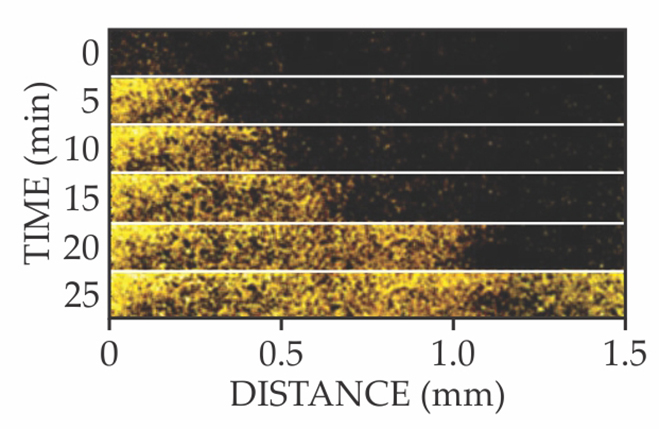
The added extracellular K+ changes the peripheral cells’ membrane potentials and hinders them from absorbing glutamate. Glutamate then becomes available to the interior cells, the ion channel closes, and the cycle starts over.
Süel notes that the signaling mechanism in B. subtilis is similar to a slowly moving wave of depressed membrane potentials in the brain, which has been associated with migraines. Both involve extracellular K+, and both are triggered by metabolic stress. “In a sense, the bacteria in biofilms communicate like neurons in the brain.”
The inner workings of the YugO channel—how it opens and closes in response to glutamate shortage or extracellular K+—are still largely a mystery. In addition, the researchers want to see if other bacterial species use similar electrochemical communication.
Another avenue that Süel wants to follow is to see if K+ signals travel beyond a single colony. In their work, they observed that extracellular K+ concentrations extend past the biofilm edge. Potentially, the K+ signal could reach cells or other colonies not in direct contact with the biofilm.
References
1. A. Prindle et al., Nature 527, 59 (2015). https://doi.org/10.1038/nature15709
2. J. Liu et al., Nature 523, 550 (2015). https://doi.org/10.1038/nature14660




