How squid build their graded-index spherical lenses
DOI: 10.1063/PT.3.3718
The deep ocean is a dim place. The amount of sunlight that reaches a depth of 300 m is roughly 1/10 000 of that at the surface. Squid have adapted to those low-light aquatic conditions by evolving eyes with lenses that are nearly spherical in shape. A spherical lens has the shortest possible focal length for a given lens radius. It thus optimizes the size of the eye opening relative to the size of the image it makes on the retina. Unlike the oblong human lens, which changes shape to change focal length, the squid lens has a fixed focal length. Squid focus by moving the lens. But a spherical lens can suffer spherical aberration, and the greater the focusing power, the more serious the aberration.
In 1854 James Clerk Maxwell showed theoretically that a spherical lens could be made aberration free if its refractive index varied parabolically as a function of the distance from the lens’s center. Evolution beat Maxwell to the discovery by millions of years: Squid, such as the one seen in figure
Figure 1.

The squid eye up close. To optimize the refractive power of their eyes without suffering spherical aberration, squid have evolved sphere-shaped lenses with a radial gradient in the protein density and, thus, the refractive index. The inset shows schematically the continuous protein-density gradient in the spherical squid lens. The dashed circles at relative radii of 40%, 60%, and 80% separate the spherical layers into which lenses were sectioned for experiments.
© 2014 MBARI

Alison Sweeney and her colleagues at the University of Pennsylvania now report that they may have a solution to the long-standing puzzle of how the squid lens establishes its protein-density gradient in a way that maintains uniform transparency. 1 They found that cells at different radial positions within the lens produce different ratios of some 40 subtly different variants of S-crystallin. All the mixtures form gels—or at least a volume-spanning protein network—but at varying densities. The gelation prevents the proteins from aggregating into opaque clumps and damps local density fluctuations that could distort vision.
Evolved self-assembly
The S-crystallin variants in the squid lens share a common motif. Each variant has a roughly spherical, globular body and two flexible rabbit-ear loops. The loops differ in length, from three amino acids to more than a hundred. And the loops’ amino-acid sequences are wildly varied.
As a graduate student during the mid 2000s, Sweeney had approached the problem of the squid lens from an evolutionary-biology perspective. She already knew that the many different S-crystallins are evolutionary relatives of an enzyme, glutathione S-transferase, that helps to neutralize toxic molecules. Selective pressure should prevent so many mutated copies. “Evolution tends to prune things out,” she explains. But Sweeney’s investigations showed that natural selection had maintained the production of all those variants. That finding indicated that the structural variation might serve some important function. 2
The salient physics, says Sweeney, is about self-assembly. By the time a squid eye is fully formed, lens cells have expelled their nuclei and ribosomes, leaving S-crystallins to account for more than 95% of the remaining nonwater mass. Somehow, the proteins have self-assembled inside cells at the right densities so that the refractive index of the lens varies from 1.3 at the edge, matching seawater’s refractive index, to 1.6 at the center. At the lens’s core, the proteins are squished in so tightly that water has been entirely expelled from the cells. At the periphery, the lens density is close to that of water.
When Sweeney arrived at the physics department at the University of Pennsylvania in 2012, she decided to tackle the physics of the lenses. Postdoc Jing Cai recalls that her first assignment was to catch squid. Once the squid were caught, Cai sectioned their squid lenses into four annular layers, as sketched in the inset of figure
The lenses prepped, Sweeney and her colleagues performed RNA sequencing and found 53 unique messenger-RNA transcripts for S-crystallin. They can’t be sure how many of those become unique proteins. However, electrophoresis showed that S-crystallins of various molecular weights are present in each lens layer (figure
Figure 2.

Protein variants combine in radially varying ratios in the lens of a squid eye. The gray curves show the measured relative molecular-weight distributions of the proteins in the four annular lens layers shown in the inset. The colored peaks are components of a Gaussian fit that takes into account the molecular weights of the variants determined from RNA-sequencing studies. The lower panels show structure models derived from small-angle x-ray scattering data for each layer. At the periphery of the lens, the proteins form loose chains with each protein linked to two or, occasionally, three others. At the core, the proteins are tightly packed, and each protein is joined to four or more others. (Adapted from ref.
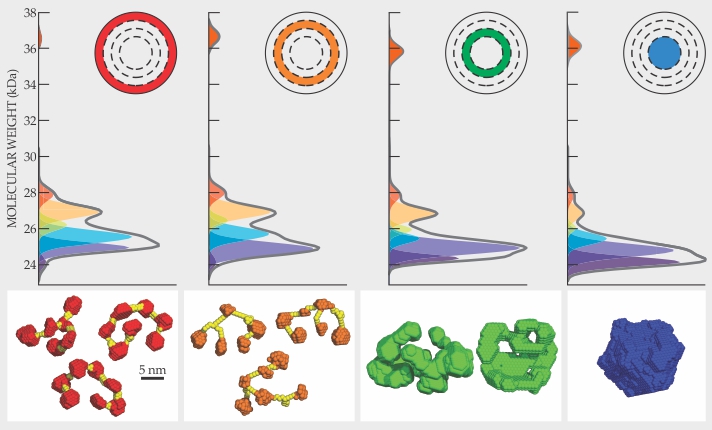
The researchers performed small-angle x-ray scattering (SAXS) measurements to investigate the kinds of protein structures present. Sweeney’s group had gone into the SAXS experiments expecting to find evidence for repulsive interactions that would prevent proteins from forming opaque clumps. With purely repulsive interactions, the scattered x-ray intensity should fall off at low scattering angles. What the researchers observed instead was scattered intensity that decreased continuously with increasing angles, a pattern caused by attractive interactions of particles that touch one another to form larger effective particles.
Century eggs and gels
The group was struggling to understand the attractive interactions when Erika Eiser of Cambridge University happened to visit the University of Pennsylvania. She had been investigating the egg-white transformation in the century egg, a Chinese delicacy prepared by burying a raw egg in alkaline mud for weeks to months. In the alkaline mud, the yolk turns dark green and the white turns into a brown translucent gel.
Eiser noticed the similarities between attractive interactions she had found in century eggs, which she had interpreted using patchy colloid theory, and those in Sweeney’s squid lenses; she suggested that Sweeney take a look at a paper on patchy colloid theory by Francesco Sciortino’s group at the University of Rome. 3 Cai says Sweeney came to her “saying let’s try these patchy particles. And it totally worked.”
Patchy colloid theory deals with spherical colloidal particles with sticky surface patches through which they interact. Particles with bigger or more numerous patches are able to interact with more neighbors than those with fewer or smaller patches. The theory can be applied to the squid lens by treating individual proteins’ globular bodies as the colloidal particles and their rabbit-ear loops as the sticky patches.
At the periphery, particles with long loops predominate; they tend to make loop–loop links. Molecular dynamics simulations showed that two loops from neighboring proteins attach to each other via hydrogen bonds. That situation can be represented by particles with two patches. Moving inward, the loops become shorter, and, occasionally, loops from three proteins simultaneously join so that the proteins form branching chains, which could be represented by particles with three patches. At even shorter loop lengths, the loops can attach to the bodies of other proteins to form more densely packed branching networks. Those cases would be represented by particles with more than three patches. At the core, proteins completely fill the volume and individual proteins might be stuck to four or more others.
The theory predicts that patchy colloid particles naturally link up into chains even at low particle concentrations, which explains an experimental difficulty the Pennsylvania researchers encountered. Typically, one would dilute the proteins, separate protein types, and run scattering experiments on isolated proteins in solution to characterize their scattering properties. But that wasn’t possible with the S-crystallins. “No matter how much we diluted the proteins in the squid lens, we always had chains,” explains Sweeney.
One knock against the Pennsylvania group’s work could be that their analysis of x-ray data used a single, averaged form factor, which describes the scattering properties of individual proteins, whereas the sample contained many S-crystallin variants, each with its own form factor. However, Sweeney and Cai maintain that the form factors don’t vary much between variants and that trying different individual ones didn’t change their conclusions.
Sweeney’s group speculates that as the lens grows and new cells are added, they are genetically programmed to make particular ratios of the S-crystallin variants. According to the theory, patchy particles become gels when the particle density reaches some threshold determined by the number of patches on the constituent particles. When that gelation occurs, messenger RNA can no longer move about the cell, and protein production stops. At the lens periphery, the predominance of long-looped proteins results in an average of about two patches per particle. At the core, a greater number of short-looped proteins means more patches per particle. The larger the number of patches, the denser the protein packing. The consequent radially varying protein density leads to a gradient in the refractive index.
Sweeney notes that the squid lens appears to be the first natural system to manifest properties predicted by patchy colloid theory. She asks, “What can we learn from that natural system?”
References
1. J. Cai et al., Science 357, 564 (2017). https://doi.org/10.1126/science.aal2674
2. A. Sweeney et al., J. R. Soc. Interface 4, 685 (2007). https://doi.org/10.1098/rsif.2006.0210
3. E. Bianchi et al., Phys. Rev. Lett. 97, 168301 (2006). https://doi.org/10.1103/PhysRevLett.97.168301




