High-frequency electrolysis begets spontaneously combusting nanobubbles
DOI: 10.1063/PT.3.1319
Conventional wisdom holds that a microscopic bubble of gas, no matter how reactive its contents, makes for a stubborn explosive. That’s because explosive combustion sustains itself on recycled energy: Reacting molecules generate heat, which in turn incites more molecules to react, and so on. Due to its large surface-to-volume ratio, however, a microscopic bubble tends to lose heat to its surroundings so fast that the chain of reactions stops before it ever truly gets going.
Few people are more familiar with that problem than Vitaly Svetovoy, Miko Elwenspoek, and their colleagues at the University of Twente in the Netherlands. Looking to develop a novel type of fast and powerful electrochemical actuator, they fashioned an array of microelectrodes that, supplied with alternating voltage pulses, could extract micron-sized bubbles of hydrogen and oxygen from water. But their varied attempts to ignite the bubbles were to no avail.
On increasing the pulse frequency past 20 kHz, however, the team made a counterintuitive discovery: Although the microbubbles were apparently too small to combust, nanobubbles weren’t. 1 In fact, not only did nanobubbles combust, they did so spontaneously.
According to prevailing theory, that shouldn’t be possible—at least not at the ambient conditions used in the team’s experiment. Crucially, however, the pressure inside each nanobubble was likely much higher than the atmospheric pressure maintained in the lab. That’s because the Laplace pressure, the excess internal pressure due to surface tension, is large for small, highly curved bubbles. In the instants just after nucleation, a bubble’s Laplace pressure can be thousands of atmospheres. By the time the bubble grows to a few tens of microns across, its Laplace pressure dwindles to nearly zero.
Above 20 kHz, each electrode sees a full pulse cycle—a negative pulse that extracts H2 and a positive pulse that extracts O2—in a matter of microseconds. That means a bubble nucleated during the initial negative pulse can acquire a reactive mixture of H2 and O2 before it reaches 100–200 nm in diameter, while its Laplace pressure is still as high as 30 atm. Svetovoy and his colleagues suspect it’s that high pressure, along with the fast dynamics associated with the short time scales, that creates the conditions for autoignition.
Not seeing is believing?
The combusting bubbles were too small to be seen, existed for only a few microseconds, and never emitted a flame. Confirming that the tiny explosions did in fact occur proved a challenge.
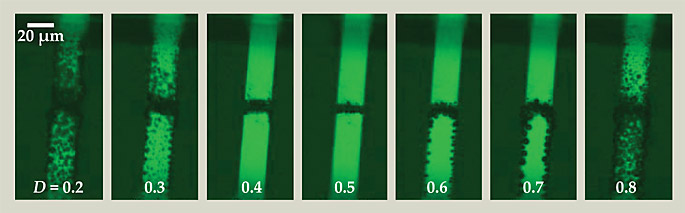
A case can be built in part from the images shown in the figure. Taken after 200 µs of operation at 100 kHz, they show that when positive and negative pulses are applied with unequal durations—to produce either mostly O2 or mostly H2—the electrodes quickly become coated with microbubbles. The same is true when electrodes are supplied with only positive or negative pulses. Only when electrodes are supplied with alternating pulses of roughly equal duration—to extract a two-to-one mixture of H2 and O2—do they fail to generate visible bubbles. Says Svetovoy, “The only explanation that seemed plausible was that the bubbles were being consumed by chemical reaction.”

Supplied with high-frequency alternating voltage pulses, the electrodes shown here extract tiny bubbles of hydrogen and oxygen gas from the surrounding water. If the alternating pulses are of roughly equal duration—that is, if the duty cycle D is near 0.5—newly nucleated bubbles acquire a reactive mixture of gases while still small enough to spontaneously combust. (D gives the fraction of time that the polarity of the bottom electrode is negative.) If the alternating pulses are applied disproportionately, H2 and O2 form in unequal proportions and the bubbles grow into stable, visible microbubbles. (Adapted from ref.
Laser vibrometry measurements add rigor to that argument. Capable of detecting slight fluctuations in light’s optical path between a source and a reflecting surface, vibrometers are most commonly used to detect mechanical motion. But with the vibrometer trained on the relatively still electrodes, the changes it detects in the optical path can be attributed to variations in the refractive index of the intervening fluid: Nucleation of low-refractive-index gas bubbles shortens light’s roundtrip, whereas depletion of bubbles lengthens it. Provided the bubbles are smaller than about 200 nm, so that they don’t scatter light, the vibrometer serves as a sensitive indicator of the gas concentration above the electrode.
Consistent with the reaction interpretation, vibrometry measurements show that the gas concentration increases and decreases in phase with the alternating voltage in the moments after the high-frequency pulses begin. Further evidence of combustion included a slight temperature increase near the electrodes, which could be attributed to exothermic reactions but not resistive heating, and noticeable wear on the electrode surface. That all of the behaviors depended on the ratio of H2 to O2 led the team to rule out purely mechanical mechanisms such as cavitation.
Satisfied that high-frequency electrolysis does incite nanocombustion, the researchers have now shifted their focus to finding ways to use the new effect. Experts anticipate applications ranging from catalyst-free fuel cells to cleaning devices for nanostructured materials. Svetovoy, for his part, envisions a nanobubble-powered microscopic internal combustion engine. “But that’s still a long way off,” he says. “For now, it’s still a dream.”
References
1. V. B. Svetovoy, R. G. P. Sanders, T. S. J. Lammerink, M. C. Elwenspoek, Phys. Rev. E 84, 035302 (2011). https://doi.org/10.1103/PhysRevE.84.035302




