Force spectroscopy unveils hidden protein-folding states
DOI: 10.1063/PT.3.3542
For 30 years atomic force microscopy (AFM) has been imaging surfaces with subnanometer resolution. As a thin, flexible cantilever with an atomically sharp tip is scanned back and forth across the surface, the tip’s vertical position is monitored by reflecting a laser beam off it. The cantilever’s minute deflections reveal the surface topography.
But AFM is not limited to determining where atoms and molecules are—it can also manipulate them. In single-molecule force spectroscopy, for example, an AFM tip attached to one end of a protein, as shown in figure
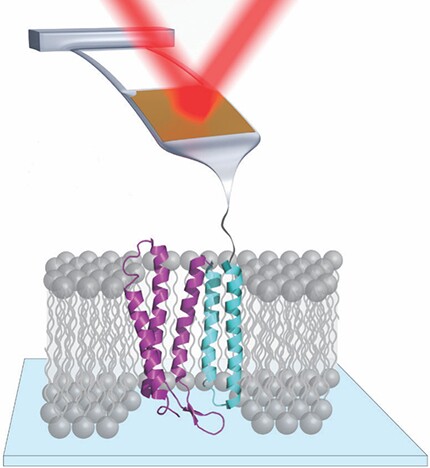
Figure 1. In single-molecule force spectroscopy of the membrane protein bacteriorhodopsin, the tip of an atomic force microscopy cantilever is attached to the protein. As the cantilever is raised, it gently tugs on the protein, and its vertical position is tracked by a reflecting laser beam. Bacteriorhodopsin consists of seven vertical helices; for clarity, only five are shown here.
JILA
In contrast to the atomic-resolution surface images, AFM measurements of protein-folding dynamics have offered only a coarse view. The problem is water: Proteins need to be studied in an aqueous environment, and hydrodynamic drag slows a standard cantilever’s response time to hundreds of microseconds. Any change in the protein’s state that occurs on a faster time scale is invisible to such an approach.
For the past decade, Thomas Perkins and colleagues at JILA in Boulder, Colorado, have been working to develop a cantilever optimized for studying protein folding with high time resolution. Now the JILA researchers have put their most advanced cantilevers to work to probe the folding dynamics of bacteriorhodopsin, a protein that harvests photon energy to pump protons across the cell membrane. 2 With 10 times the force precision and 100 times the time resolution of previous AFM measurements of bacteriorhodopsin, they’ve uncovered an intricate protein-folding landscape that had never been seen before.
All that glitters
The perfect cantilever for force spectroscopy needs several qualities. It should move through water easily enough to respond to a microsecond change in the protein’s state. It should be supple enough that a piconewton force produces a measurable deflection. It should be overdamped, so a change in the applied force doesn’t set it oscillating. It should be optically reflective, so the laser signal can be detected. And it should be stable over the course of an experiment, so a given force always produces the same deflection.
Sometimes those requirements are at odds with one another. The easiest way to reduce hydrodynamic drag is to make the cantilever shorter, but that also increases stiffness and reduces damping. Commercial cantilevers come coated in gold to maximize their reflectivity. But Perkins and company discovered that—for reasons not entirely understood—the gold causes the cantilever’s free-standing position to drift over time, which destabilizes force measurements. 3
Figure

Figure 2. A commercial cantilever in an aqueous environment creates too much hydrodynamic drag for microsecond-resolution force spectroscopy, and its gold coating causes force measurements to drift over time. To overcome both those problems, Thomas Perkins and colleagues modify the cantilevers with the quick four-step protocol shown here. (Adapted from ref.
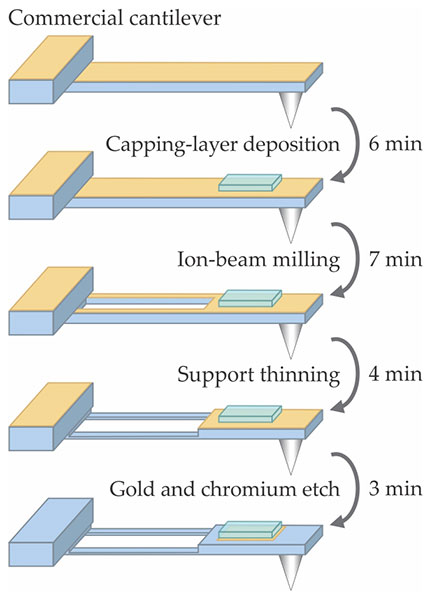
Thus modified, a short (40 µm) cantilever can be used in a standard AFM with no problems. But when Perkins and colleagues progressed to modifying ultrashort (9 µm) cantilevers, they found that the remaining area of gold was too small to reflect the laser beam. So they engineered a new detection-laser module for their AFM system to focus the laser to a sufficiently small spot. 5
Bacteriorhodopsin
Although the JILA researchers performed proof-of-principle tests along the way to check their modified cantilevers’ time resolution and force precision, the new work represents their first move toward a system of biophysical interest. Bacteriorhodopsin, a protein in the cell membrane of saltwater-dwelling single-celled organisms, is homologous to G-protein coupled receptors (GPCRs), an important class of membrane proteins in more complex organisms, including humans. The receptors are responsible for many types of cellular signaling, and as such, they’re the targets of some 40% of all pharmaceutical drugs.
But the dynamics of GPCRs and other membrane proteins are not well understood, for several reasons. They tend to be larger than the more commonly studied globular proteins, and their structure and function depend critically on the heterogeneous environment of the cell membrane. Unlike many smaller proteins that find their correct folded structures on their own, membrane proteins fold via an intricate process that often requires the assistance of so-called chaperone molecules.
Like GPCRs, bacteriorhodopsin consists of seven helical structures that extend across the membrane. Previous force spectroscopy studies typically found intermediate states that corresponded to the full unfolding of each of those helices. But molecular dynamics simulations predict that many more intermediates should exist.
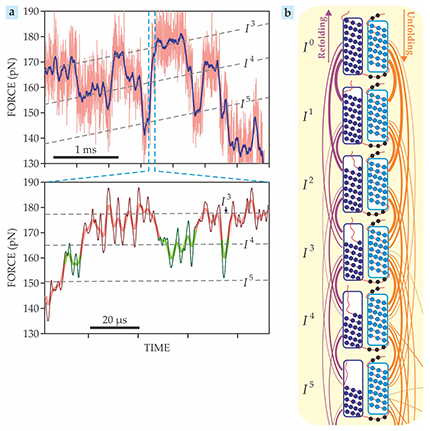
With their improved cantilevers, Perkins and colleagues studied the bacteriorhodopsin helices shown in blue in figure

Figure 3. Unfolding two helices of bacteriorhodopsin revealed 14 stable intermediate states (In for n = 1–14, with I0 representing the fully folded helices), most of which had never been detected before. (a) Force–time plots show the molecule hopping among the third, fourth, and fifth intermediates, indicated by the dashed lines. As highlighted by the green segments in the bottom panel, sometimes intermediates are occupied for just a few microseconds. (b) The first five intermediates, schematically shown here, span the unfolding of half of the first (dark blue) helix. The orange and purple curves indicate the likelihood of various unfolding and refolding transitions. (Adapted from ref.
The surfeit of data is just the beginning; just what it all means remains to be seen. Comments Michael Woodside of the University of Alberta in Edmonton, “As we’ve seen repeatedly in other contexts, an order-of-magnitude increase in resolution often leads to big changes in understanding of the underlying physics. I expect something similar to happen here.”
Off the shelf
Perkins and colleagues’ modified AFM apparatus isn’t the only way to obtain a microsecond view of biomolecular folding. Last year, Woodside and colleagues achieved similar resolution by tethering each end of a molecule to a bead held in an optical trap. (See Physics Today, June 2016, page 14
And Perkins is hoping for a day when researchers won’t have to make those modifications themselves. “None of our cantilever improvements are covered by a patent or pending patent,” he says, “and there’s no reason why cantilever companies and AFM manufacturers can’t implement 90% of them using wafer-scale manufacturing.” In the short term, he’s collaborating with other groups to demonstrate the cantilevers’ scientific applicability; in the longer term, he hopes those studies will spur demand for manufacturers to commercialize the modified cantilevers.
References
1. P. E. Marszalek et al., Nature 402, 100 (1999). https://doi.org/10.1038/47083
2. H. Yu et al., Science 355, 945 (2017). https://doi.org/10.1126/science.aah7124
3. A. B. Churnside et al., Nano Lett. 12, 3557 (2012). https://doi.org/10.1021/nl301166w
4. M. S. Bull et al., ACS Nano 8, 4984 (2014). https://doi.org/10.1021/nn5010588
5. D. T. Edwards et al., Nano Lett. 15, 7091 (2015). https://doi.org/10.1021/acs.nanolett.5b03166
More about the authors
Johanna L. Miller, jmiller@aip.org




