Five-molecule water clusters have liquid-like properties
DOI: 10.1063/pt.yqgq.rfum
How big is a drop of water? That’s not an unanswerable question, like “How long is a piece of string?” A single H2O molecule isn’t a drop. As more and more molecules cluster together, there must be some threshold at which they start displaying behaviors associated with the condensed phase.
Nor is the question a mere philosophical curiosity. How many water molecules make a drop is relevant to the chemistry of such environments as Earth’s atmosphere and interstellar space, where molecules are typically found in isolation or in small clusters. It’s also relevant to computational chemistry: Simulating molecules takes a lot of computing power, so modelers want to know how many molecules they really need to reproduce liquid water’s properties.
How does one even tell whether water is acting like a bulk liquid? One thing that condensed-phase water can do, but single molecules can’t, is dissolve other substances; as a paradigmatic case, the research community has settled on hydrogen chloride. High school chemistry students learn that HCl is a so-called strong acid: When dissolved in water, its molecules always break up into H+ and Cl− ions, with the former latching onto water molecules to make H3O+. On its own, though, HCl is a covalently bonded molecule. So how much water is needed to split it into an ion pair?
At the German Electron Synchrotron (DESY), Melanie Schnell and colleagues are now shedding some light on that question. 1 She and her postdocs Fan Xie, who worked on the experimental side of the study, and Denis Tikhonov, who handled the theory, have conducted one of the most detailed and thorough studies yet on HCl microhydration. They found that in clusters of four water molecules, HCl is intact, but with five water molecules, it splits into a pair of ions. Although “five” isn’t a universal answer to “How many water molecules make a drop?”—different amounts of water might be needed under different conditions and to dissolve different substances—the work is a step toward demarcating the boundary between the molecular and condensed-phase worlds.

Water droplets come in various sizes. But how small can they be before they stop acting like liquids? (Image by Aathavan jaffna/Wikimedia Commons/CC BY-SA 3.0

Standing on shoulders
Water-cluster structures can be hard to elucidate. There’s no way to zoom in and snap a photo of a single cluster to see whether an HCl molecule is dissociated. Some techniques come close to directly visualizing the atom-by-atom structures of molecules (see Physics Today, December 2017, page 22
Importantly, though, “enormous” is not “infinite.” In the cold environment of the neon jet, clusters tend to settle into structures that are local energy minima. Just like molecules, those cluster structures can be calculated with density functional theory (DFT) or similar methods. And just like molecules, the clusters have discrete rotational and vibrational quantum states, which means they can be probed spectroscopically.
The strongest spectral lines are those of single molecules and dimers: The molecules can arrange themselves in fewer ways, so each structure creates a larger share of the signal. As researchers figured out the structures of larger clusters by matching the spectral lines with their computed DFT predictions, those lines could be subtracted from the spectrum. What was left was a forest of weaker and more numerous lines from larger and more complicated clusters. 2
It’s been a decades-long quest by many research groups to untangle the IR (vibrational) and microwave (rotational) spectra of water clusters, with and without HCl or other guest molecules, and Schnell acknowledges that her group’s work builds on previous efforts. “In particular, Zbigniew Kisiel’s studies on smaller HCl–water clusters significantly eased the challenges in our analysis of larger clusters,” she says. “Our work would have been much more difficult and tedious without those pioneering efforts.”
Spin splitting
Several factors allowed Schnell and colleagues to progress from studying clusters with three or four water molecules to those with five or more. First, they used a high-resolution microwave spectrometer, which sampled the spectral range from 2 to 8 GHz with a resolution of 25 kHz, so they could distinguish spectral lines that previous lower-resolution IR studies could not. 3
Second, their DFT search for cluster structures included not just the energy-minimizing structures themselves but also the pathways between those minima. Many of the structures that the researchers calculated were similar in energy and had similar predicted rotational spectra. Only by understanding how the structures might interconvert could the researchers figure out which structures were actually showing up in the spectrum.
Third, they exploited an interaction between rotational quantum states and nuclear spin. Both of chlorine’s stable isotopes, 35Cl and 37Cl, have electric quadrupole moments, which impart a hyperfine splitting to spectral lines. But the splitting is dampened when the nucleus is surrounded by a complete electron shell, as in a Cl− ion. When Cl is part of an H–Cl covalent bond, on the other hand, the spin is only partially blocked by electrons, and the splitting is much larger. By measuring the magnitude of hyperfine splitting, Schnell and colleagues found valuable clues to figure out which cluster structure they were looking at—and, crucially, whether the HCl molecule was intact or dissolved.
None of those factors by itself is new. The nuclear-spin effect, in particular, was described by Charles Townes and Benjamin Dailey back in 1949. 4 But they’d never been applied together to the H2O–HCl cluster system. “Before our studies, the search for HCl dissociation with a few water molecules was almost there,” says Schnell. “We just gave it a final kick.”
Four, five, six, seven
Acid–water clusters come in three types, as shown in figure
Figure 1.

In a cluster of water molecules, a hydrogen chloride molecule can dissociate into a separated ion pair (left) or a contact ion pair (center), or it can remain undissociated (right). In four-water clusters, such as the ones shown here, only the intact HCl structures were observed. But with five or more water molecules, the H–Cl bond can break. (Adapted from ref.
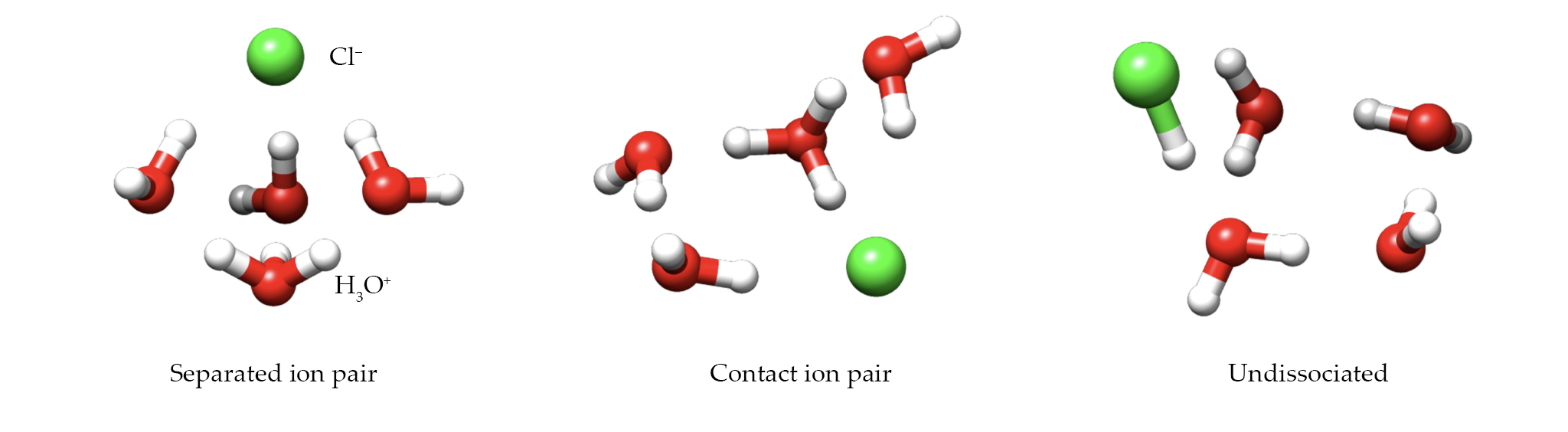
Schnell and colleagues didn’t observe any separated ion pairs. At first, they were surprised and disappointed, but when they looked more closely at their DFT calculations, the result made sense. All of the energy barriers to forming those structures were too high for clusters to surmount in the cold environment of the neon jet.
The analysis therefore came down to distinguishing contact ion pairs from undissociated structures, and the Townes–Dailey nuclear-spin effect was crucial. The two classes of structures can be hard to differentiate from the arrangements of atoms alone, because shifting an H atom a fraction of an angstrom from an HCl molecule to an H3O+ ion might not have much of an effect on the cluster’s rotational energy levels. But it makes all the difference to the hyperfine splitting.
From the splitting that they measured, the DESY researchers calculated the clusters’ ionic character on a scale from 0 (for a nonpolar covalent bond, as in a Cl2 molecule) to 1 (for a Cl− ion). As figure
Figure 2.

Between four and five water molecules in a cluster, there’s an abrupt transition in which a hydrogen chloride molecule dissociates from a covalently bonded molecule into a pair of ions. The chlorine’s ionic character is quantified on a scale from 0 to 1 by the Cl nuclear spin’s effect on hyperfine splitting. When the Cl atom is part of a molecule, the splitting is relatively large and the ionic character is low. But in a free Cl− ion, the nucleus is shielded by a complete electron shell, which dampens the splitting almost to zero. (Adapted from ref.
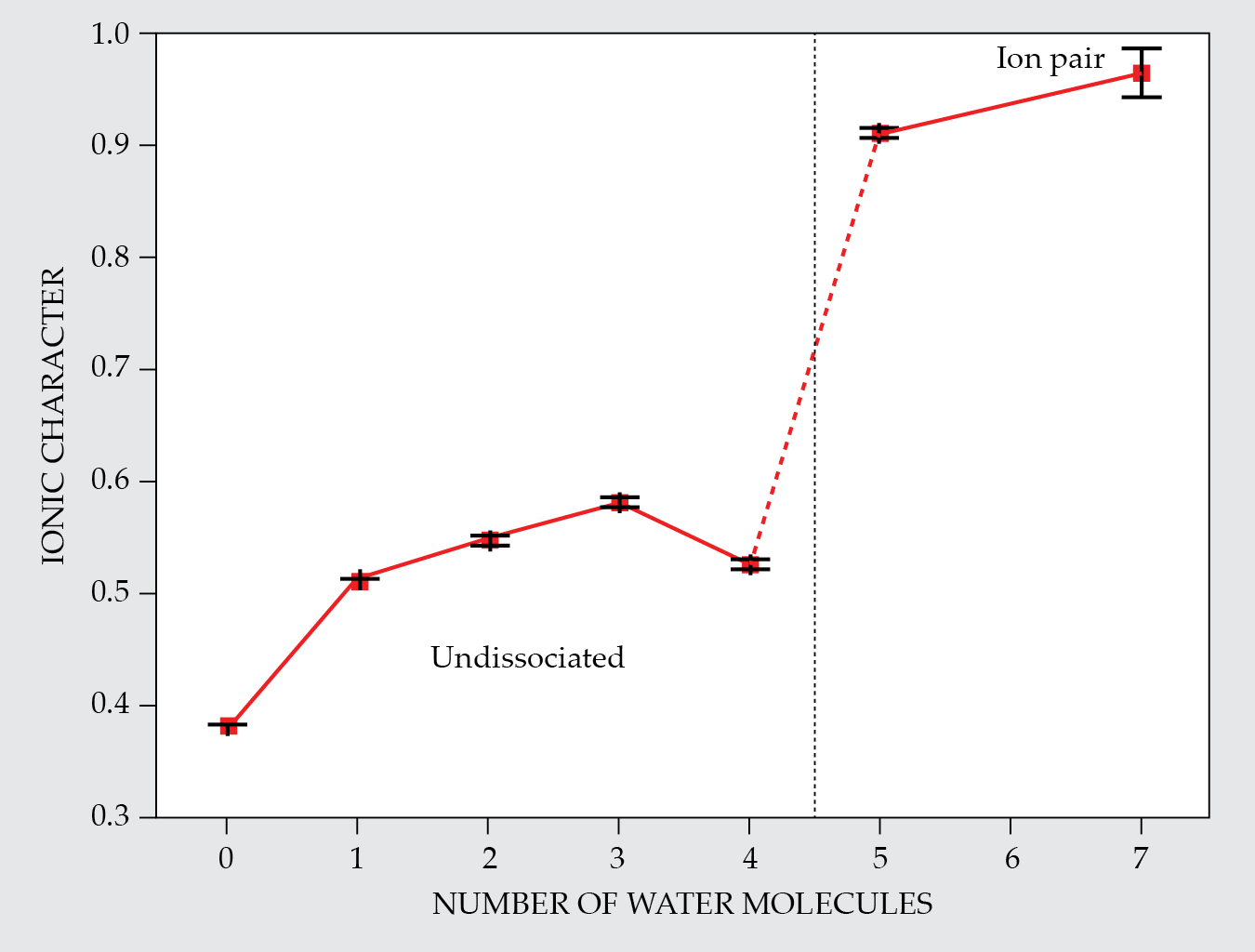
What about clusters with six water molecules? The researchers have every reason to think that those will also form contact ion pairs, but they’re still working on the spectral assignments. Perhaps counterintuitively, six-water clusters are harder to study than seven-water clusters, because they tend to have lower symmetry. With five or seven water molecules, the Cl− and H3O+ ions can sit in the middle of the cluster, with the rest of the H2O molecules arranged in pairs or trios symmetrically around the edges. With six water molecules, there’s no such option, and the lower-symmetry clusters give rise to more complicated spectra.
The more pressing questions are the ones the researchers don’t know the answers to. Can the clusters be made to form separated ion pairs, not just contact ion pairs, either by adding more water molecules or changing the experimental conditions? Do other acids behave the same way as HCl? Spectroscopic study of acid–water clusters is not going to get any easier, as the simplest remaining structures have their spectral lines assigned. But with the right combination of experimental and theoretical tricks—including the Townes–Dailey nuclear-spin effect—further progress may be possible. “Nuclear spin is really cool,” says Xie. “It can reveal molecular properties that are difficult to assess in other ways.”
References
1. F. Xie, D. S. Tikhonov, M. Schnell, Science 384, 1435 (2024). https://doi.org/10.1126/science.ado7049
2. See, for example, Z. Kisiel et al., J. Phys. Chem. A 104, 6970 (2000). https://doi.org/10.1021/jp001156o
3. D. Schmitz et al., J. Mol. Spectrosc. 280, 77 (2012). https://doi.org/10.1016/j.jms.2012.08.001
4. C. H. Townes, B. P. Dailey, J. Chem. Phys. 17, 782 (1949). https://doi.org/10.1063/1.1747400
More about the authors
Johanna L. Miller, jmiller@aip.org




