Electron diffraction sees hydrogen atoms
DOI: 10.1063/PT.3.3483
For finding the atomic structures of new molecules and materials, x-ray crystallography remains the reigning technique of choice. But it’s hampered by a major limitation: Its simplest and most powerful form requires a crystalline specimen tens of microns on a side—a size that’s beyond crystal growers’ reach for many substances. The size requirement results from two factors: the relatively weak scattering of x rays off atoms in the crystal and the impracticality of focusing x rays onto a submicron spot.
Electrons, because of their charge, scatter orders of magnitude more strongly than x rays do, and they can be focused much more tightly. Electron diffraction, therefore, has the potential to solve the structures of submicron crystals, which are much easier to grow than larger ones. But the strong scattering has also been electron diffraction’s drawback: Electrons typically change direction many times on their way through even a thin crystal, and their diffraction patterns are extremely difficult to interpret quantitatively.
For more than 20 years, researchers have been working, with increasing success, toward antidotes to that multiple scattering. Now Lukáš Palatinus (Czech Academy of Sciences in Prague), Philippe Boullay (CRISMAT Laboratory, CNRS, Caen, France), and their colleagues have reached a milestone: using electron diffraction to derive structures that explicitly include the positions of hydrogen atoms. 1
Shown in figure

Figure 1. Structural models of (a) paracetamol and (b) framework cobalt aluminophosphate, derived from electron-diffraction data. Non-hydrogen atoms are shown explicitly, and the hydrogen-atom positions are established from electrostatic potential peaks. Large peaks are shown in yellow and smaller ones in gray; in each case, the largest peaks agree well with the known or expected locations of H atoms. (Adapted from ref.
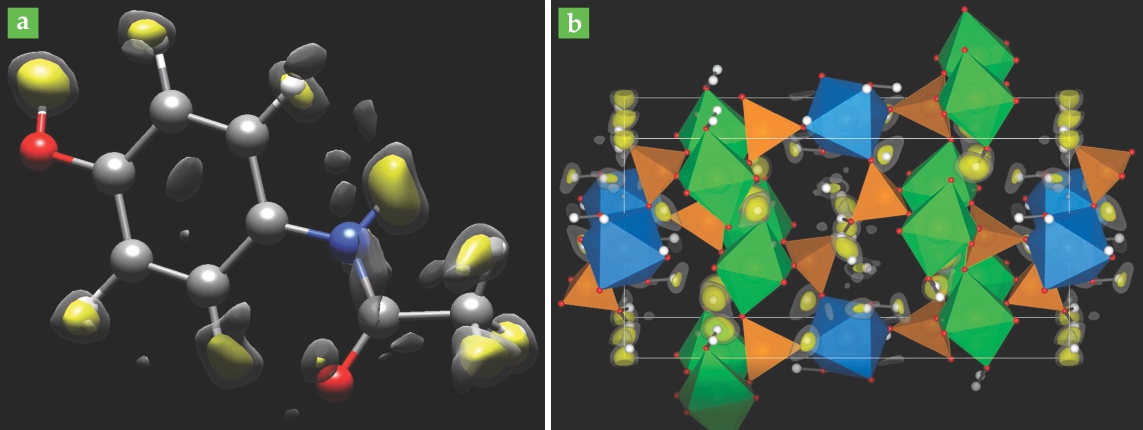
Humble hydrogen
In any form of crystallography except special neutron-based methods, hydrogen atoms are difficult to see: Their low scattering power and large vibrational amplitudes combine to produce broad, weak signals that are easily overwhelmed by noise. Crystallographers typically have to guess at the H-atom positions once they’ve solved for the positions of all the heavier atoms. Much of the time, that approach works well. Organic molecules, for example, have characteristic bond lengths and angles that don’t usually change much from molecule to molecule.
But H-atom positions can sometimes offer important insights into a material’s chemistry. When an H atom breaks away from its molecule, for example, it often does so as a bare proton, leaving a negatively charged ion behind. So the positions of H atoms—and how closely they’re bound to the surrounding heavier atoms—can be intimately related to a molecule’s electric charge distribution, which in turn is related to its solubility in water and other solvents. And solubility is an important property to know for many substances, not least for newly discovered drugs to be administered in solid form.
That said, locating H atoms wasn’t the Prague–Caen researchers’ explicit or immediate goal. “It was more like a dream come true than the initial motivation for the experiment,” says Boullay. Rather, he and his collaborators sought to continue the steady march of electron diffraction progress.
Off-axis tilt
An early milestone on that march was reached in 1994, when Roger Vincent and Paul Midgley introduced precession electron diffraction (PED). 2 In x-ray diffraction, aligning the beam with one of the crystal’s major axes yields a diffraction pattern with a multitude of peaks, each directly related to the intensity of scattering off a single crystal plane. But in electron diffraction, the strength of multiple scattering means that the one-to-one relationship between peaks and planes no longer holds. Furthermore, electron diffraction patterns are extremely sensitive to sample thickness, small crystal imperfections, and slight misalignments of the beam.
In PED, the electron beam is tilted by a degree or two—the angle is exaggerated in figure

Figure 2. (a) In precession electron diffraction (PED), the electron beam is tilted and precessed about a desired beam axis; the diffracted beams are recombined and their intensities are integrated to produce a diffraction pattern. (Omitting the detilting step would yield overlapping circles instead of discrete diffraction peaks.) (b) Without PED, multiple scattering muddies the relationship between peak intensity and crystal structure, and diffraction patterns are difficult to interpret. (c) With PED, the brightest peaks correspond to the strongest crystal reflections. (Panels b and c adapted from P. A. Midgley, A. S. Eggeman, IUCrJ 2, 126, 2015.)
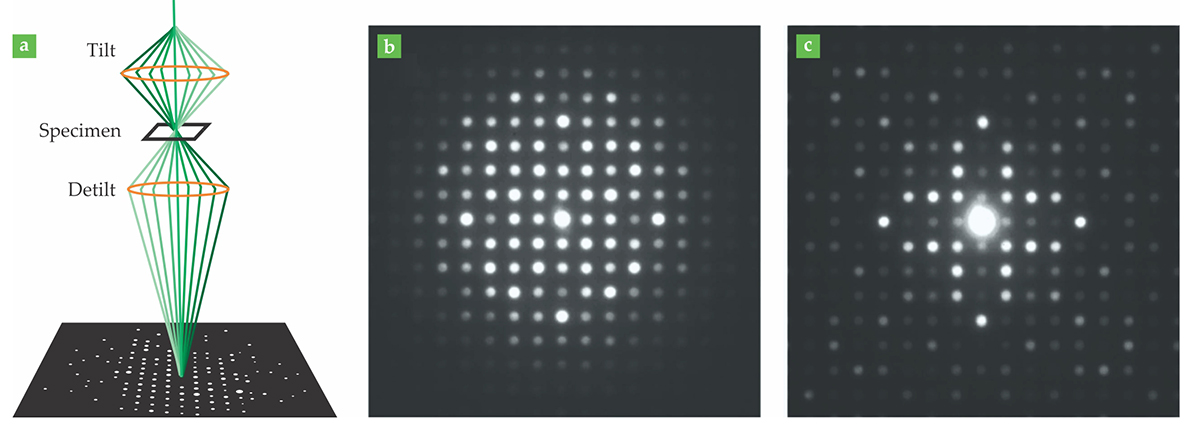
Although PED has been around for more than 20 years, it’s caught on only in the past decade or so, since the technology has become commercially available to perform the precession with the beam of an electron microscope. Also around 10 years ago, Ute Kolb and her colleagues at Johannes Gutenberg University in Mainz, Germany, began work on the complementary technique of electron diffraction tomography. 3 Often combined with PED, EDT mitigates multiple scattering by directing the electron beam far away from the crystal axes. With the beam at an arbitrary off-axis angle, it can reflect off only a few of the many crystal planes, and the number of available multiple-scattering pathways is greatly reduced.
But EDT has the disadvantage that structures can no longer be solved with just a few diffraction patterns. Instead, data at dozens, hundreds, or even thousands of different beam angles may be necessary, depending on the complexity of the material. “The new electron diffraction methods have benefited greatly from digital cameras replacing photographic film,” says Sven Hovmöller, whose group at Stockholm University also worked on the development of EDT. “Collecting hundreds of diffraction patterns with film would not only have been too expensive, but it would have taken days to collect the exposures and then weeks to scan them all.”
Many structures have been solved with the combination of PED and EDT. “But the accuracy and reliability of the structures was variable and nearly impossible to estimate,” says Palatinus. “The errors on the positions of atoms could be as low as 0.03 angstroms, but it’s not exceptional to have errors of up to 0.5 angstroms”—around ⅓ the length of a chemical bond.
Mapping potential
Better accuracy requires dynamical scattering theory to explicitly model and account for multiple scattering. There are various ways to do that—for example, the sample can be numerically simulated slice by slice, or the electrons can be treated as quantum mechanical Bloch waves. Palatinus and colleagues’ approach uses a Bloch-wave treatment to iteratively refine the structure and check it against diffraction data. They first published their computational method in 2013, and in 2015 they adapted it to accommodate EDT off-axis data. 4
The latest advance combined PED, EDT, the dynamical scattering codes, and the experimenters’ perseverance. Each frame in an EDT series requires the sample to be irradiated by the beam for several seconds; most materials, especially organic ones, can’t survive the exposure necessary for a full data series. What’s more, it’s not always clear when the beam is starting to damage the crystal. In their work on cobalt aluminophosphate, the Prague–Caen researchers collected a whole EDT series before realizing that the sample was showing signs of deterioration. They started over with a new sample and lower beam intensity; they eventually solved the structure using 578 diffraction patterns from six separate crystals. For paracetamol, which can tolerate just 30 seconds in the electron beam before breaking down, they manually shifted the sample between frames so that different parts of the crystal were being irradiated over the 52-frame series.
The structures in figure
The new results show that when x-ray diffraction fails due to crystal size, electron diffraction has the potential to stand in for it. But Palatinus and Boullay don’t expect x-ray diffraction to give up its position as the favored technique for structural analysis anytime soon. The lower risk of radiation damage to the sample makes x-ray diffraction far less technically challenging. And although the dynamical scattering theory calculations can be performed on an ordinary desktop computer, they can take up to several hours to complete; computing structures from x-ray diffraction data is 100 to 1000 times faster.
References
1. L. Palatinus et al., Science 355, 166 (2017). https://doi.org/10.1126/science.aak9652
2. R. Vincent, P. A. Midgley, Ultramicroscopy 53, 271 (1994). https://doi.org/10.1016/0304-3991(94)90039-6
3. U. Kolb et al., Ultramicroscopy 107, 507 (2007). https://doi.org/10.1016/j.ultramic.2006.10.007
4. L. Palatinus et al., Acta Crystallogr. A 69, 171 (2013);https://doi.org/10.1107/S010876731204946X
L. Palatinus, V. Petříček, C. A. Corrêa, Acta Crystallogr. A 71, 235 (2015). https://doi.org/10.1107/S2053273315001266
More about the authors
Johanna L. Miller, jmiller@aip.org




