Diamond defects enable nanoscale nuclear magnetic resonance
DOI: 10.1063/PT.3.1934
Understanding a protein’s function is difficult if you don’t know its structure. The standard tools of the trade, x-ray crystallography and bulk nuclear magnetic resonance (NMR), work well for molecules that can be produced in macroscopic quantities and arrayed in a crystal or dissolved in solution. But for many proteins and other biomolecules, those requirements have proven prohibitive.
If shrunk down to the nanoscale, magnetic resonance imaging (MRI), the common medical imaging technique, could offer a powerful tool for probing molecules that don’t crystallize or dissolve. Inspired by that approach, Daniel Rugar and colleagues at IBM’s Almaden Research Center in San Jose, California, developed magnetic resonance force microscopy (see Physics Today, September 2004, page 21
Now two teams have taken a first step toward room-temperature nanoscale MRI by making NMR measurements of nanometer-sized volumes. One team was led by a collaboration 2 of Rugar and David Awschalom (University of California, Santa Barbara). The other was led by Friedemann Reinhard and Jörg Wrachtrup of the University of Stuttgart in Germany. 3 Both teams used nitrogen–vacancy (NV) centers—point defects in diamond best known for their promise as qubits, the potential building blocks of an eventual quantum computer—as sensitive magnetometers.
Magnetic resonance
Bulk NMR and MRI are two different ways of gaining information about a sample from the resonance frequencies of magnetic nuclei (hydrogen-1, carbon-13, and others) in a magnetic field. That resonance frequency, in the RF, corresponds to the energy splitting between nuclear spin states, which depends on the nuclear gyromagnetic ratio and the local magnetic field felt by a nucleus. In bulk NMR, the goal is to pick up the subtle internal magnetic fields that combine with an applied field and affect the total field. Those internal fields, from surrounding electrons and nearby nuclei, give information about the chemical environment of the nucleus and thus the structure of the molecule.
On the other hand, MRI uses magnetic resonance signals to determine the spatial distribution of nuclei with more or less identical chemical environments—1H nuclei in water, for example. In an inhomogeneous applied magnetic field, nuclei in different locations show up at different frequencies, so a frequency spectrum provides spatial information.
Simply by shrinking the conventional MRI apparatus, researchers can resolve features as small as a few microns, but so far no smaller. To image the subnanometer features inside single molecules, new tools are needed.
Enter the NV center, a point defect in diamond that comprises a nitrogen atom (in place of a carbon atom) adjacent to a lattice vacancy. NV centers are well studied for their spin coherence properties: The defect’s spin-1 electronic ground state can be prepared in a specific superposition of its ∣0〉, ∣1〉, and ∣−1〉 sublevels and left there for many hundreds of microseconds, uninfluenced by any noise from its environment. When excited with an optical-frequency laser, the ∣0〉 state is 30% more likely to fluoresce than the ∣1〉 or ∣−1〉 state. That offers an easy way to read the NV center’s state, at least in an experiment that can be repeated enough times to accumulate sufficient statistics.
Those characteristics make NV centers good qubits. They also make them sensitive and tiny magnetometers. When an NV center initially in the ∣0〉 state is hit with a so-called π/2 microwave pulse, it is transferred into an equal superposition of the ∣0〉 and ∣1〉 states. The relative phase of those two components evolves at a rate proportional to the local magnetic field. When the superposition is “undone” with an opposite π/2 pulse, the probability that the NV center returns to the ∣0〉 state depends on the total accumulated phase. In a suitably designed experiment, that phase can be made to depend on the quantity of interest: the abundance of nearby atomic nuclei with a given resonance frequency.
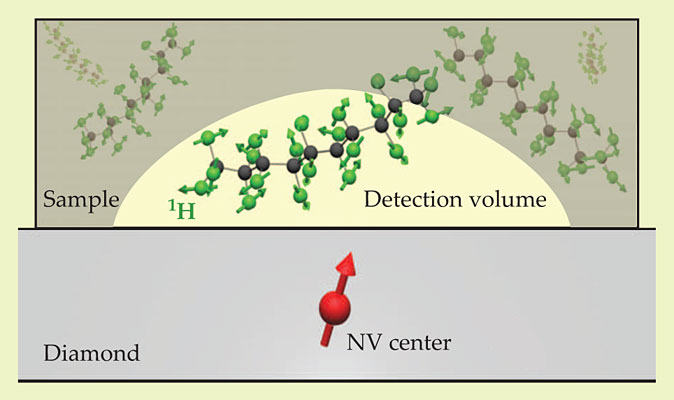
Already, NV-center NMR has been used to detect 13C nuclei elsewhere in the diamond itself. To be useful for applications, though, nanoscale MRI needs to be sensitive to nuclei in arbitrary samples outside the diamond. Toward that end, both teams used an experimental setup like the one in figure 1: An NV center a few nanometers beneath the diamond surface could respond to the 1H spins in an organic sample just above the surface. The dimensions of the detection volume roughly equal the NV center depth: 20 nm for the California team’s experiment, 5 nm for the Stuttgart group’s. In all cases, when the sample was removed, the 1H signal went away.

Figure 1. A nitrogen–vacancy (NV) center a few nanometers beneath a diamond surface can sense the tiny magnetic fields of the hydrogen-1 nuclei in a nearby organic sample. (Adapted from ref.
Frequency and time
The California team’s setup included a microwire, lithographically added to the diamond surface, that could generate both RF pulses to manipulate the sample’s nuclear spins and microwave pulses to manipulate the NV center’s electronic spin. That allowed them to do a spin-echo measurement, as shown in figure 2a, reminiscent of conventional NMR. Midway between the π/2 pulses, they applied a microwave π pulse (with a different polarization) that essentially reversed the sign of the phase accumulated during the first half of the sequence. If the field felt by the NV center is the same throughout the sequence, the total accumulated phase at the end should therefore be zero, so an opposite π/2 pulse should reliably return the NV center to the ∣0〉 state.

Figure 2. A frequency-domain spin-echo measurement. (a) The laser and microwave (MW) pulses at the beginning of the sequence prepare the nitrogen–vacancy (NV) center in a superposition of its ∣0〉 and ∣1〉 states. The pulses at the end measure the final relative phase of the ∣0〉 and ∣1〉 components. Only if the frequency fNMR of the intermediate RF pulses equals the resonance frequency of the sample nuclei is that phase nonzero. (b) As expected, there is a dip in the signal at a frequency proportional to the applied magnetic field B. (Adapted from ref.
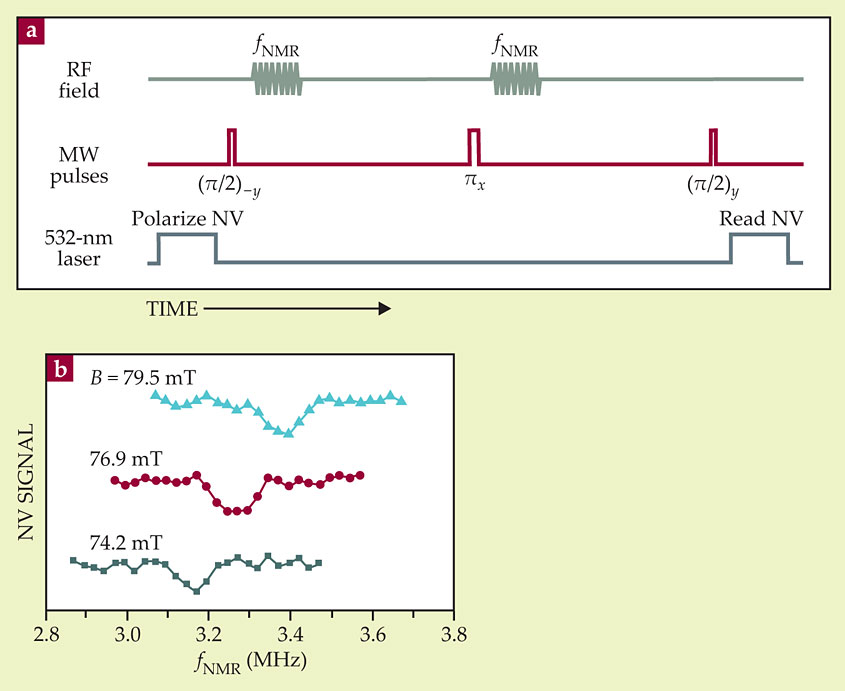
But along with the π pulse, they applied an RF pulse. If that pulse is out of resonance with the 1H nuclei in the sample, it has no effect. But if it’s in resonance, it flips the spins of the sample nuclei, so the field felt by the NV center is not the same during the two halves of the sequence. (To guard against the possibility that the RF pulse has some spurious effect on the NV state, they applied an identical pulse at the beginning of the sequence.) From the NV final state as a function of the RF pulse frequency, they should thus be able to detect the sample protons’ resonance frequency.
Sure enough, as shown in figure 2b, they saw a dip in the spin-echo signal, the position of which was proportional to the applied magnetic field. As expected, the constant of proportionality was the gyromagnetic ratio of the 1H nucleus.
The Stuttgart group’s setup lacked the microwire on the diamond surface. They used an external resonator to create the microwave pulses to drive the NV center, but they aren’t yet able to manipulate the sample spins. Instead, they designed a sequence of microwave pulses that, placed between the two π/2 pulses, created an accumulated phase that’s sensitive to the spontaneous precession of the randomly oriented spins. The sequence comprises about 100 π pulses, equally spaced by a delay τ. By varying τ and then transforming their data from the time domain into the frequency domain, they found a signal peak corresponding to the sample nuclei’s resonance frequency.
The California team could do time-domain measurements as well, by breaking their RF pulses into two equal halves separated by a delay τ. The half pulses interfered either constructively or destructively, depending on whether τ was a multiple or half multiple of the resonant period (see the article by Norman F. Ramsey in Physics Today, July 1980, page 25
What lies ahead
Detecting a single 1H peak in a nanometer-sized sample is just the first step, and nanoscale MRI is still a long way off. To produce images, the researchers need a way to take measurements at different points in the sample. They could apply an inhomogeneous magnetic field, as in conventional MRI. Or they could attach the NV center to a scanning probe tip. The Stuttgart researchers have already built the setup for the latter approach, Reinhard says, “so I hope the first proof of principle won’t take too long.”
But the bigger challenge, says Rugar, lies in improving the signal-to-noise ratio enough to detect small numbers of nuclei. At present, the California team’s detection volume contains about a million nuclei; the Stuttgart group’s, about 10 000. Moving the NV center even closer to the diamond surface would shrink the detection volume, although it would also reduce the defect’s coherence time. More efficient detection of the fluorescent photons that reveal the NV center’s state would also help. The California researchers estimate that with realistic improvements in both those areas, imaging a molecule atom by atom is a goal within reach.
References
1. C. L. Degen et al., Proc. Natl. Acad. Sci. USA 106, 1313 (2009). https://doi.org/10.1073/pnas.0812068106
2. H. J. Mamin et al., Science 339, 557 (2013). https://doi.org/10.1126/science.1231540
3. T. Staudacher et al., Science 339, 561 (2013).https://doi.org/10.1126/science.1231675
More about the authors
Johanna L. Miller, jmiller@aip.org




