Dangling DNA pinpoints a protein’s chemical groups
DOI: 10.1063/PT.3.2738
X-ray crystallography and electron microscopy can each provide a wealth of structural information about proteins and other biomolecules. But neither technique can operate under physiologically relevant conditions, so they’re unable to observe the dynamic processes that are central to proteins’ biological function. Atomic force microscopy, on the other hand, can work in water. Now Duckhoe Kim and Ozgur Sahin of Columbia University have used that capability to create a specialized atomic force microscope (AFM) that can locate specific chemical groups in a protein under biological conditions. 1
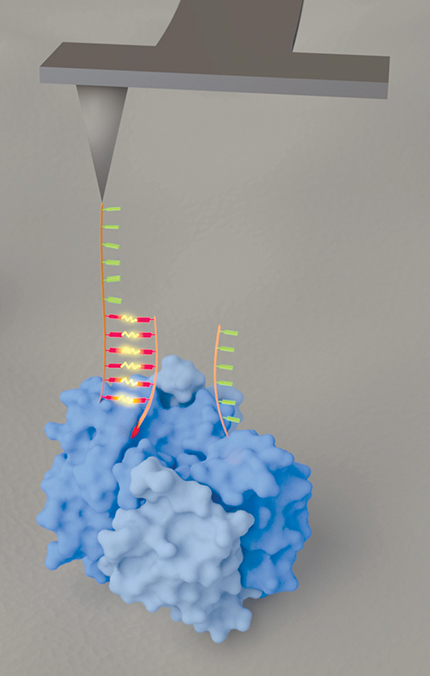
From their AFM tip the researchers dangle two single-stranded DNA hexamers, shown in figure 1 in red and green. The complementary strands, shown in like colors, are attached to a protein’s chemical groups of interest and serve as imaging labels. Whenever one of the hexamers comes into contact with its complement, the two strands briefly bind, and the AFM cantilever is observed to deflect—a signal that one of the chemical groups has been found.

Figure 1. An atomic force microscope outfitted with two DNA hexamers, shown in red and green, one dangling from the end of the other. The complementary strands, shown in like colors, are attached to chemical groups of interest in a protein (blue). When one of the hexamers at the end of the tip locates its complement (and thus the associated chemical group), the two strands briefly bind, and the T-shaped cantilever is seen to twist. (Courtesy of Ozgur Sahin.)
The information provided from a single scan with the device is fairly sparse: just the positions of the two labels. But in principle, one could re-image the same protein again and again, with different chemical groups labeled each time, and build up a full chemically specific three-dimensional structure from the resulting pairwise distances. To study dynamic conformational changes, on the other hand, detailed structural information might not be necessary. Tracking the positions of two labels over time may suffice.
Tip taps
Outfitting AFM tips with biomolecules has been done before. 2 But previous approaches have used large, bulky molecules—such as whole proteins and their antibodies—that bind so strongly that they have to be ripped apart by the force of the AFM cantilever. In contrast, vertically hanging DNA has a small horizontal footprint. And paradoxically, the new method’s success lies in the weakness of the binding force between the DNA hexamers. A stronger force, even between slightly longer DNA strands, could induce distortion in the protein that would muddy the signal. But shorter strands bind only when they’re precisely aligned, and they readily unbind as the cantilever is moved away.
To give each hexamer the chance to find its complement—and to allow the tip itself to acquire a conventional AFM image—the researchers operated the microscope in tapping mode, with the cantilever set to vibrate up and down at its resonance frequency. Binding of the two hexamers would occur at different parts of the oscillation cycle, so they can be distinguished.
But with a standard cantilever, a binding event induces a vertical pulling force—that is, a force along the direction in which the cantilever is already vibrating. That collinearity makes it hard to separate the soft tug of the DNA from the baseline sinusoidal oscillation of the cantilever. To better distinguish the different components of the motion, Kim and Sahin used a T-shaped cantilever, as shown in figure 1, that Sahin and his colleagues had devised a few years ago. 3 The tip still vibrates vertically, but now DNA binding causes the cantilever to twist. With the vertical and twisting motions tracked separately, the binding of the two hexamers can be more readily detected.
To test the technique’s resolution, the researchers attached many copies of the complementary hexamers directly to a solid substrate, then searched for them with the AFM. They found that binding events formed clusters less than 0.5 nm in diameter, so they took that value to be an estimate of the microscope’s resolution. “That means,” explains Sahin, “that two spots separated by more than 0.5 nm are likely to represent two objects.”
Finding biotin
For their proof-of-principle experiment, Kim and Sahin looked at the streptavidin–biotin complex. Streptavidin is a bacterial protein that accommodates four copies of the small nonprotein molecule biotin, also known as vitamin B7. The system is advantageous for two reasons. First, the structure of the whole complex has been well determined by x-ray crystallography. Second, biotin molecules with custom DNA sequences already attached are commercially available. Says Sahin, “That makes sample preparation relatively simple.” Kim and Sahin bought some DNA-bound biotin, incubated it with streptavidin molecules that they’d affixed to a solid substrate, and imaged the resulting complexes with their AFM.
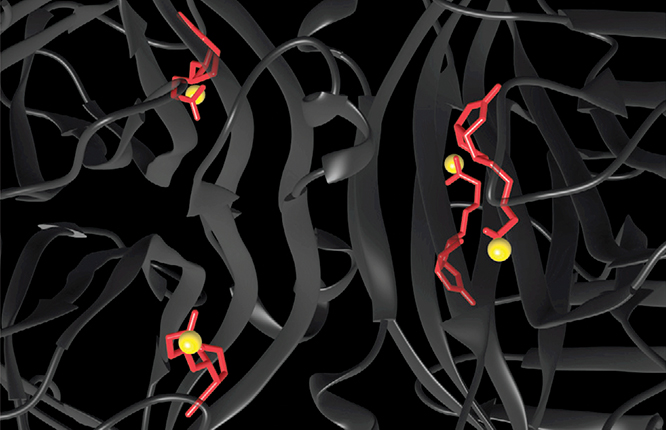
From imaging a large number of randomly oriented complexes, the researchers found that the distances between DNA-labeled biotins in the same complex clustered around three values: 3.0 nm, 5.3 nm, and 6.0 nm. Three pairwise distances are what’s expected if the four biotins form a tetrahedron with each pair of opposite edges the same length (as is known to be the case). Kim and Sahin calculated the shape of the tetrahedron, superposed it on the protein structure, and calculated where the molecules’ ends must be, as shown by the yellow spheres in figure 2. The AFM-derived positions agree with those in the crystal structure to within 0.2 nm.

Figure 2. The crystal structure of the streptavidin–biotin complex, with the streptavidin protein structure shown in dark gray and the biotin molecules shown in red. The yellow spheres represent the AFM-derived positions of the ends of the biotin molecules—in agreement with their crystal-structure positions to within 0.2 nm. (Adapted from ref.
To make the best use of their method, Kim and Sahin would like to be able to apply a DNA label to any part of a protein molecule on demand. But that’s no easy feat, in part because proteins are made up of many copies of the same amino acids, with little to chemically distinguish them. In the meantime, the researchers are turning their attention to naturally occurring biomolecular complexes between proteins and DNA or RNA. “The RNA strand that is part of the complex may provide the label that we need,” says Sahin, “so they may be easier to study. We are exploring that possibility.”
References
1. D. Kim, O. Sahin, Nat. Nanotechnol. 10, 264 (2015). https://doi.org/10.1038/nnano.2014.335
2. See, for example, P. Hinterdorfer, Y. F. Dufrêne, Nat. Methods 3, 347 (2006). https://doi.org/10.1038/nmeth871
3. O. Sahin et al., Nat. Nanotechnol. 2, 507 (2007); https://doi.org/10.1038/nnano.2007.226
M. Dong, O. Sahin, Nat. Commun. 2, 247 (2011).https://doi.org/10.1038/ncomms1246
More about the authors
Johanna L. Miller, jmiller@aip.org




