Atoms on a surface quickly slip through crowds
DOI: 10.1063/PT.3.4198
Most industrial chemicals are the products of catalysis. A catalyst’s efficacy depends not only on its ability to facilitate and accelerate reactions but also on its ability to mix with reactants and spread, which, on a surface, is determined by lateral diffusion. Despite the importance of surface diffusion, researchers have yet to observe and understand all its basic atomic-scale dynamics.
Early diffusion studies focused on clean or sparsely populated surfaces with quick diffusion rates. But when the density of atoms increases or there are two or more different species on the surface, as is often the case in industrial processes, diffusion becomes more complicated. Intuitively, one expects higher density and the presence of other particles to slow down diffusion as the adsorbed species get in each other’s way. But that doesn’t have to be the case.
Joost Wintterlin of the Ludwig-Maximilians University Munich and his colleagues have taken a step toward tackling more complicated diffusion dynamics. Using high-speed, variable-temperature scanning tunneling microscopy (STM), they observed a previously unidentified mechanism that allows for surprisingly rapid diffusion of atoms on a surface crowded with molecules. 1
Diffusion mechanisms
When an atom is alone on a surface, it settles into a low-energy binding site. To move around, it receives a temporary boost to its energy through thermal fluctuations and hops from binding site to binding site. For a higher density of atoms or two species on a surface, diffusion suffers two complications: First, particles must compete for binding sites in an effect called site blocking. Second, particles attract or repel one another. Those factors alter and limit the types of motion available to an atom.
A few different types of lateral diffusion occur on covered surfaces. One common mechanism in two dimensions, and the most prominent in 3D diffusion, is the vacancy mechanism. As atoms jostle on a surface, vacancies between them change position. A given atom can move only when a vacancy gets close to it. Because the atom must sit and wait for a vacancy, the process is often slow. A second mechanism, known as direct exchange, was observed for the first time 10 years ago on a chlorine-terminated silicon surface with some Cl replaced with hydrogen. 2 In direct exchange, two adjacent atoms swap positions. By repeating that process, the Cl atom randomly walks its way across the surface. In a related ring-exchange mechanism, a sulfur atom on a chlorine-covered copper surface exchanges sites with Cl atoms in a ring through a rotation by a site. 3
However, it was not clear whether any of those three mechanisms apply to diffusion on a catalyst surface. The allowed motions necessarily change in systems with different types of bonding to the surface and different particle interactions.
Another door opens
In the course of their research on the fundamental steps of catalytic reactions, Wintterlin and his colleagues developed and combined two STM operational capabilities—high speed and variable temperature—to observe short-time-scale dynamic processes over a wide range of relevant activation energies. Whereas standard STM takes on the order of a minute to complete one image, high-speed STM completes 50 frames in a second. To achieve the increase in speed, the researchers scan the tip at a constant height rather than the usual method of maintaining a constant current, which requires a delay for the feedback loop.
Variable-temperature STM is difficult to perform because the positioning piezoelectric crystal is sensitive to thermal drift. Wintterlin and his colleagues measure between 50 K and 500 K with a beetle-type STM, in which three piezo legs hold the STM tip and scanning piezo. The instrument reduces horizonal thermal drift with its near-cylindrical symmetry and reduces vertical drift because the expansion or contraction of the legs compensates for that of the scanning piezo. Although those capabilities are not unique to Wintterlin’s group, the marriage of the two is uncommon.
Ann-Kathrin Henss, the first author on the study, recorded 10 STM images per second of an oxygen atom on a ruthenium surface covered with a saturation layer of carbon monoxide molecules, shown schematically in figure
Figure 1.

Two types of atomic motion. (a) On a ruthenium surface (gray), an oxygen atom (orange) trapped by a hexagonal ring of carbon monoxide molecules (blue) can move only among the three sites at the center of the hexagonal cage. (b) By moving out of position (green arrow), a CO molecule opens the door for the O atom to escape (orange arrow). The CO molecules then rearrange into a new hexagonal pattern, as indicated by blue arrows. (Adapted from ref.
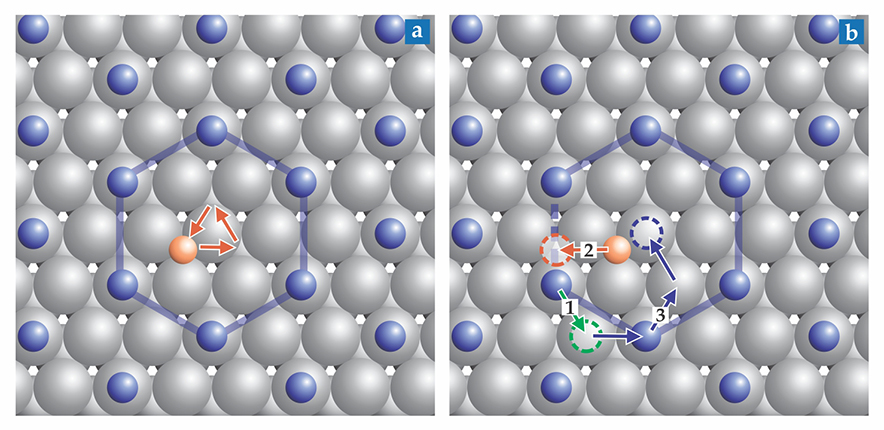
Figure
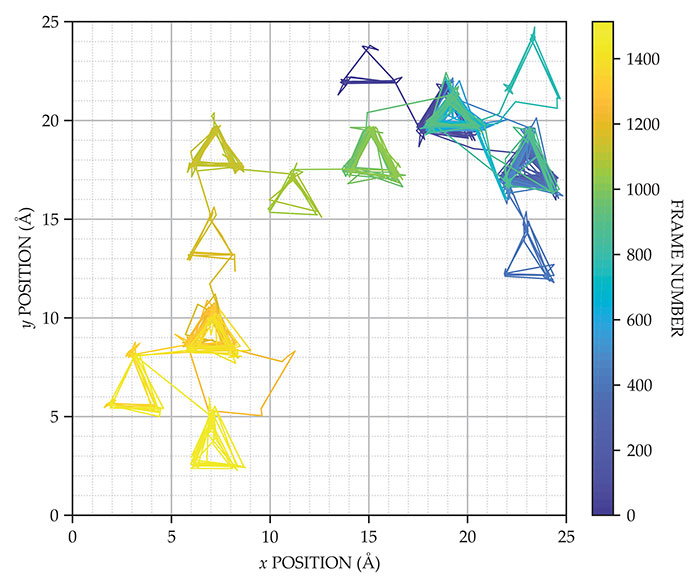
Figure 2.

Trajectory of an oxygen atom on a highly covered ruthenium surface, as measured by high-speed scanning tunneling microscopy, is plotted as a function of frame number. The triangular patterns arise from the trapping of the O atom by hexagonal cages of carbon monoxide molecules. Despite the high density of CO molecules on the surface, the O atom moves tens of angstroms in two minutes. The measurements were taken at 12 frames per second at 273 K. (Adapted from ref.
Making the first move
The experimental time resolution wasn’t fast enough to show whether the O and CO move simultaneously, the O moves first, or the CO moves first. Axel Gross of the University of Ulm in Germany and his colleagues joined the project to answer that question through density functional theory calculations. They found that the total activation energy was high for concerted motion and the oxygen-initiated scenario, whereas thermal fluctuations of the CO density easily sufficed to free the O atom.
A CO molecule moves from its post on the hexagonal ring to a site next to an adjacent CO molecule, as shown by the green arrow in figure
Surprisingly, the door-opening diffusion mechanism is almost as fast as the diffusion of O on a bare surface. But atomic processes do not always translate directly into macroscopic diffusion constants. Disorder, defects, and particle–particle interactions influence macroscopic diffusion and perhaps explain why previous diffusion measurements in similar systems showed slower rates.
The situation is further complicated in catalytic reactions, crystal growth, and other chemical processes when reactions and phase transitions must be taken into consideration. It is also not yet clear how common the door-opening diffusion mechanism is. “However, the system investigated—oxygen atoms embedded in a CO layer on ruthenium—should not be special,” explains Wintterlin. “It can be generalized to strongly bound particles embedded in a layer of weakly bound other particles, adsorbed on a metal. This situation is quite general in heterogeneous catalysis.”
High spatial- and temporal-resolution in situ experiments are important for theoretical studies of diffusion as well. “One can readily provide an ab initio description of system thermodynamics,” says Jim Evans of Iowa State University, an expert in modeling nonequilibrium processes at surfaces. “But it is extremely difficult to anticipate the dominant dynamics or kinetics controlling transport processes.”
References
1. A.-K. Henss et al., Science 363, 715 (2019). https://doi.org/10.1126/science.aav4143
2. M.-F. Hsieh, D.-S. Lin, S.-F. Tsay, Phys. Rev. B 80, 045304 (2009). https://doi.org/10.1103/PhysRevB.80.045304
3. B. Rahn et al., Angew. Chem. Int. Ed. 57, 6065 (2018). https://doi.org/10.1002/anie.201712728




