An electrical insulator turns metallic within a femtosecond
DOI: 10.1063/PT.3.1873
State-of-the-art FETs can process electrical signals at rates of about 100 GHz. They can’t get much faster than that because of capacitance introduced by electrodes and resistance from the scattering among charge carriers and phonons. But it’s also possible to drive carriers ballistically, and all-optical methods for injecting current—no electrodes required—into semiconductors have led to electrical switching on picosecond time scales. Indeed, coherent oscillations of electrons driven by an external electric field were observed in superlattices 20 years ago (see the article by Emilio Mendez and Gérald Bastard in Physics Today, June 1993, page 34
An international collaboration led by Ferenc Krausz of the Max Planck Institute of Quantum Optics in Garching, Germany, and the Ludwig-Maximilians University of Munich has now demonstrated how to increase that switching rate another three orders of magnitude, into the petahertz regime. 1 , 2 What’s more, the collaboration did it using silica glass, a material long thought to be unsuitable for electrical conduction. With their large gap between valence bands and conduction bands, dielectrics offer a fast response to an applied field; but they also inevitably break down or melt when exposed to the strong electric fields required to excite electrons across the gap. The trick to transforming silica from insulator to conductor, the researchers realized, is to shine a light pulse on it that’s intense enough to overcome the bandgap and short enough to avoid damaging the material.
In a series of experiments, they exposed silica to a train of visible light pulses, each just 4 fs in duration and amplified to a peak intensity near 1014 W/cm2. Such a strong pulse generates an electric field of more than 2 V/Å—comparable to that binding silicon and oxygen atoms in the solid—and it utterly reshapes the band structure. As the electric field approaches that strength, silica’s valence- and conduction-band levels become dramatically shifted and fan out by several eV, an effect known as Wannier–Stark ladder formation. Filled valence-band and empty conduction-band states originally separated by 9 eV approach each other to within a couple eV and are pulled into resonance with the oscillating laser field.
Mark Stockman of Georgia State University and other theorists in the collaboration reasoned that at resonance, silica’s polarizability increases dramatically and that electrons should respond to field swings by making large excursions from their lattice sites. The relocation of charge as the field turns on and off can, when configured into a circuit, generate an AC current.
Steering current
No electronic device can measure currents oscillating at optical frequencies. But there’s a way around that: Measure a net time-integrated current instead. Not only can pulses be made ultrashort and ultrastrong, they can also be made identical, emitted with the same waveform from a laser cavity. That capability allowed the researchers to precisely and reproducibly control the positions of the five or so wavecrests that fit inside the envelope of a several-femtosecond pulse. Silica responds nonlinearly as the electric field grows large. The field from the peak part of the pulse excites electrons into polarizable states and simultaneously exerts the greatest pull on those electrons, driving them in that peak field direction.
The researchers fashioned a circuit made from silica coated with gold electrodes 50 nm apart and exposed it to a series of light pulses, as illustrated in figure 1. In one experiment, the pulses (blue) were applied with their field oscillations polarized along the circuit axis—toward the electrodes. By systematically varying the carrier-envelope phase of those pulses the researchers measured a time-integrated current whose direction completely reversed with changes in phase of π radians. By contrast, pulses (red) whose fields were polarized transverse to the axis generated almost no current. Both cases confirmed the expected: Fields polarized in the direction of the circuit transfer momentum to electrons—in this case nonlinearly—and drive a current.

Figure 1. In a circuit composed of silica glass separated by two gold electrodes, incident optical fields irradiate the metal–insulator surface as either single pulses or time-delayed pairs. An incident pulse (blue) polarized in the direction of the electrodes induces a net time-integrated current whose magnitude and direction depend on the phase of the pulse’s carrier envelope. A transversely polarized pulse (red) does not, by itself, generate a current. But when applied in tandem, the pair produce a current that can be steered toward one electrode or the other via a change in timing between pulses of just half the field’s period. (Adapted from ref.
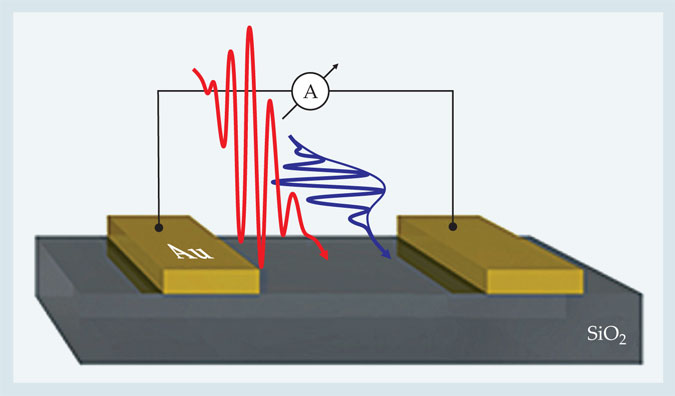
A variation of the experiment was more revealing. Rather than applying orthogonally polarized pulses independently, the researchers applied them in tandem. In that scheme, a strong transverse pulse (the red pulse in figure 1) first excited charge carriers into the conduction band, where they are more mobile. A far weaker, time-delayed pulse (blue) then drove a net current toward one of the electrodes. A change in delay time of just half the period of an optical wave reversed the current’s direction—strong evidence that the mobilization of carriers, and thus the material’s transformation from insulator to conductor, occurs within a femtosecond. 1
From the measured current density, Krausz and colleagues estimated a rise in conductivity of more than 18 orders of magnitude during that time. But to prove that it falls off just as quickly, they turned to a second type of experiment: transient optical absorption. 2 The researchers again exposed silica, this time in the form of a thin film, to visible light pulses. But they also exposed it to bursts of extreme UV (XUV) radiation, as outlined in figure 2a.

Figure 2. Attosecond spectroscopy of silica reveals modulations in the amount of extreme UV (XUV) radiation silica absorbs as a pulse of visible light passes through it. (a) Just 72 attoseconds in duration, the XUV pulse (blue) is far shorter than the light pulse (red) and can be delayed by a controllable amount to monitor the absorption at different instants in the light’s waveform. Both pulses travel from right to left. Some of the XUV photons excite a neon gas jet whose spray of photoelectrons can be used to map the light’s electric field as a function of delay (see Physics Today,
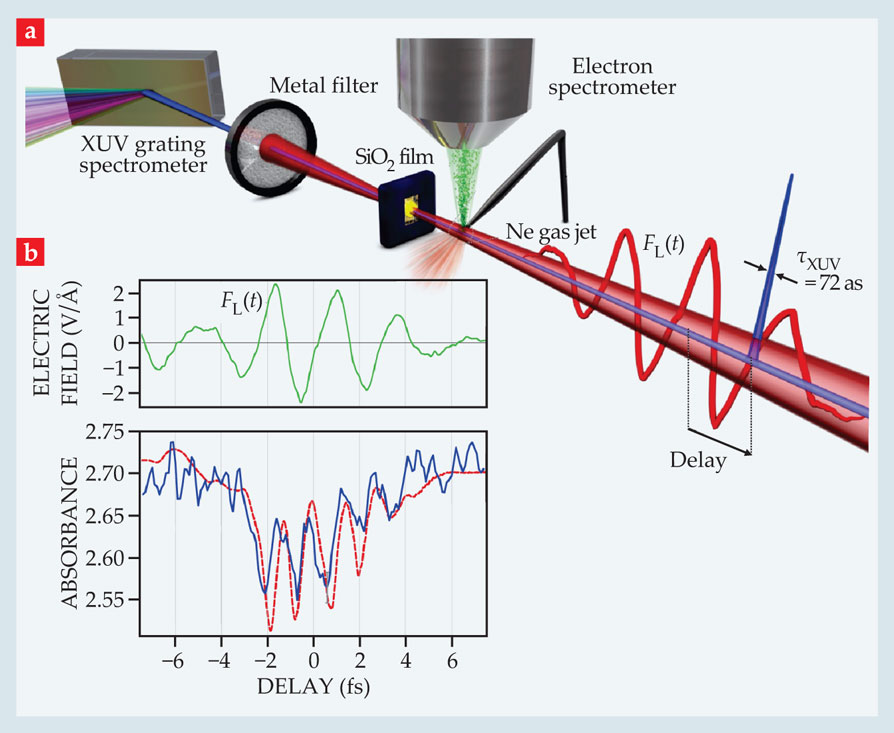
The bursts, a mere 72 attoseconds in duration (about 1/50 the duration of the light pulse), were timed so as to monitor the light’s effect on silica’s XUV absorbance at different instants within the light’s waveform. By exciting additional, deeply bound electrons from silica’s ground state into the conduction band, each XUV burst essentially takes a snapshot of the transient electronic states in the conduction band modified by the light pulse.
The light pulse’s strong field reduces the density of electron states in the conduction band, thanks to the fanning out of energy levels. At the same time, the strong field may also inject enough energy to promote electrons into the conduction band through tunneling. Both effects tend to damp the absorption of XUV photons. The former does it by reducing the absorption cross section and the latter via the Pauli exclusion principle: Electrons already in the conduction band block additional excitations to it.
Modulations in the transient absorption as a function of the XUV probe’s delay time, shown in figure 2b, turned out to be synchronous with the driving field oscillations. That observation provides evidence that the reduced cross section is the dominant effect. It also appears to confirm an ultrafast rise and fall in conductivity. Says group member Martin Schultze, “We were delighted—and surprised—that electrons don’t linger in the conduction band. Typically it takes picoseconds, or longer, for electrons to relax to the valence band. That would have made for a boring switch.”
The virtual and the real
The observation of the ultrafast fall in AC conductivity suggests that the band transitions made by electrons are virtual ones. Last year Thomas Elsaesser of the Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy in Berlin led a diffraction study of the ionic crystal lithium borohydride. 3 The group concluded that the crystal’s interaction with an intense, femtosecond optical pulse distorts the ionic potentials and generates a new quantum state that is a superposition of the valence- and conduction-band states of the unperturbed system and mimics a transition between them. The superposition state, which the group mapped by x-ray diffraction, is manifest as a shift of electronic charge from BH4− toward Li+ over the large interatomic distance between them.
According to Krausz, that sort of reversible charge relocation is likely to be closely related to his own group’s observations. The current that’s seen in an external circuit is thought to arise from a similar shift of electrons from constituent atoms closest to each metal electrode. But his collaboration can’t yet prove it based on the simplified model of a one-dimensional atomic chain.
The fields used to excite electrons in silica are so strong—some 10 times the fields used to excite LiBH4—that the material teeters on the edge of breakdown. A central question for future applications, then, is the extent to which virtual transitions are accompanied by real ones that produce a conduction-band population not reversed by the laser field. Answering that and discerning the detailed microscopic nature of the conduction is likely to require more experiments and a more realistic theory, Elsaesser says. “Silica isn’t ionic, but there’s certainly an interaction energy between O and Si. If local lattice structure matters, as it does in our work, then the picture of a 1D band used to model silica becomes tricky.”
An optically induced conductivity offers promise that electron-based signal processing may be pushed to its ultimate limit: light-speed frequencies. But don’t expect a petahertz FET anytime soon. “For that to become reality,” Krausz admits, “the full reversibility of the demonstrated femtosecond current switching cycle will have to be verified at much higher rates than the 3 kHz of the laser used in our first experiments.”
In the short term, he envisions that the team’s demonstration will bear fruit for high-speed metrology and waveform diagnostics: “A solid-state detector that records electromagnetic transients up to the frequency of visible light—a kind of petahertz oscilloscope—is pretty realistic.”
References
1. A. Schiffrin et al., Nature 493, 70 (2012). https://doi.org/10.1038/nature11567
2. M. Schultze et al., Nature 493, 75 (2012). https://doi.org/10.1038/nature11720
3. J. Stingl et al., Phys. Rev. Lett. 109, 147402 (2012).https://doi.org/10.1103/PhysRevLett.109.147402




