A new kind of self-assembled monolayer
DOI: 10.1063/PT.3.2406
The promise of self-assembled monolayers (SAMs) is far-reaching. Find a molecule that binds easily to a metal surface and, by attaching chemical functional groups to the opposite end of the molecule, you can produce a surface that combines the large-scale order of the metal with the versatility of organic chemistry. Applications range from sensors to drug delivery.
In 1983 Ralph Nuzzo and David Allara discovered that sulfur-containing molecules called thiols form SAMs on gold surfaces. (For more on the history of thiol-based SAMs and other organic thin films, see the article by Joe Greene on page 43
A class of molecules called N-heterocyclic carbenes (NHCs) is emerging as an alternative to thiols. In 2011 Ulrich Siemeling (University of Kassel in Germany) and colleagues were the first to report NHC-based SAMs on gold surfaces. 1 In 2013 Jeremiah Johnson and colleagues at MIT took the important step of showing that NHCs in a monolayer can be chemically functionalized and modified in a controlled way. 2 And now a team of researchers led by Cathleen Crudden and J. Hugh Horton of Queen’s University in Canada has characterized the stability of NHC monolayers. 3 The team found that some NHC structures could form SAMs that withstand boiling solvent, some electrochemical cycling, and pH extremes.
From atoms to surfaces
Carbenes are highly reactive molecules in which a carbon atom forms just two chemical bonds instead of the usual four. The simplest carbene is methylene, or CH2; it and most other carbenes can exist only as short-lived intermediates in a chemical reaction. But some NHCs, in which the carbene carbon is part of a ring containing two or more nitrogen atoms, are stable enough to be synthesized in bulk, purified, crystallized, and even sold commercially.
They’re still extremely reactive, though. In place of the carbene carbon’s “missing” bonds is a lone pair of electrons primed to attack a metal atom’s empty orbitals to form a strong carbon–metal bond. In principle, any carbon-containing molecule can be induced to bind to a metal, but not without first breaking one of the carbon’s existing bonds, a process with a large energy barrier. NHCs can form the same bonds with virtually no energy barrier.
Since the discovery of the first stable NHC in 1991, the chemistry of NHCs, including their ability to form complexes with isolated metal atoms, has been widely explored. “NHCs are known as privileged ligands,” explains Siemeling, “because they can bind strongly to most metals of the periodic table.” Siemeling and his group had been working on a diverse range of chemical research topics, including both NHCs and SAMs, when he got the idea to put the two together. “I surmised that NHCs should be able to bind strongly not only to the central gold atoms of molecular complexes but also to the surface atoms of solid gold substrates.”
Siemeling and his colleagues worked with an NHC similar to the structure shown in black in figure 1a. Because that molecule is capped by sturdy carbon–hydrogen bonds on every side, it can’t be chemically modified while it’s on the surface. To make a SAM that can be chemically tailored, one must replace some of the H atoms with so-called functional groups—such as halogen atoms or azide (N3) groups—before forming the monolayer. One can then use the tools of organic chemistry to modify the functional groups.

Figure 1. Two N-heterocyclic carbenes (NHCs) that bind to gold surfaces. (a) The structure shown in black, which is similar to the molecule used in the first NHC monolayer experiments,
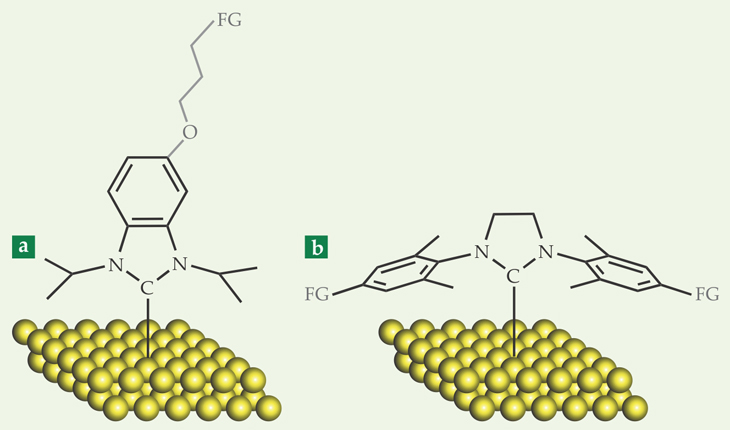
That’s what Johnson and colleagues did in their 2013 work. They attached the NHC in figure 1b, with functional groups added to the ends, to a gold surface. They then initiated a reaction on the surface-bound NHCs to create polymers of a fluorinated organic molecule where the original functional groups had been. The polymers, several nanometers long, were visible in an atomic force microscopy image.
They also used density functional theory to calculate the strength of the gold–carbon bond and found it to be about 40% stronger than the gold–sulfur bond in a thiol-based SAM. “It’s important to understand that bond strength and film stability are different things,” says Johnson. “A strong bond to the surface doesn’t guarantee a stable monolayer.” That the NHCs could withstand the eight-hour, multistep polymerization reaction without detaching from the surface was evidence of their stability.
“A new gold standard”
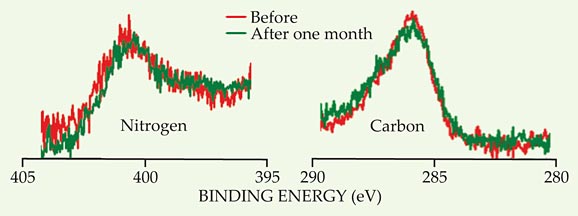
At about the same time, Crudden, Horton, and colleagues were independently postulating that NHCs could produce stable SAMs. They looked at several unfunctionalized NHC structures (although they focused on two—the one shown in black in figure 1a and a structure similar to the one in figure 1b) and a wide range of harsh conditions. They characterized their monolayers using x-ray photoelectron spectroscopy (XPS), which yields distinct peaks for the NHC’s carbon and nitrogen atoms. A decrease in the peak heights over time signals a loss of the monolayer from the surface; change in the peak shapes or positions could indicate a chemical reaction.
Overall, the NHC in figure 1a proved to be the more stable, perhaps because its lack of bulky side groups allows it to form sturdier and better ordered monolayers. Figure 2 shows the monolayer’s XPS spectra before and after being stored in water at room temperature for one month. It also survived boiling water, the organic solvent tetrahydrofuran, pH 2, and pH 12—each for 24 hours—with no measureable loss of the monolayer. But it wasn’t invincible: A 24-hour exposure to a hydrogen peroxide solution degraded the monolayer by about 20%.

Figure 2. X-ray photoelectron spectroscopy data demonstrate the stability of a self-assembled monolayer of the molecule shown in black in figure
Because one appealing application of SAMs is in electrochemistry, the Queen’s University researchers sought to test the film’s stability to electrochemical cycling. First they synthesized the functionalized NHC shown in black and gray in figure 1a and attached it to a gold surface. Then they modified the functional group to include a metal-based complex that changes its charge state in response to a change in voltage. Although the SAM was lost under negative voltage, it held up well under cycling at positive voltage, outperforming similarly functionalized thiol SAMs in that regime.
In a final series of tests, exposing an NHC monolayer to thiols had no effect: No NHCs were lost, and no sulfur-containing molecules were bound to the surface. But in the reverse situation, NHCs could completely displace a thiol monolayer. That observation may be the key to making better quality monolayers. Because NHCs are so reactive, some researchers have found that they bind not only to bare metal atoms but also to surface contaminants, locking them in place. Thiols, on the other hand, bind more selectively and are often thought to sweep away the contaminants as they do, so a contaminant-free NHC monolayer might be made simply by making a thiol-based SAM first (although Crudden and Horton have also made NHC monolayers on bare gold surfaces and see no obvious difference). “This is truly elegant,” says Siemeling. “We possibly have a new gold standard here.”
References
1. T. Weidner et al., Aust. J. Chem. 64, 1177 (2011). https://doi.org/10.1071/CH11173
2. A. V. Zhukhovitskiy et al., J. Am. Chem. Soc. 135, 7418 (2013). https://doi.org/10.1021/ja401965d
3. C. M. Crudden et al., Nat. Chem. 6, 409 (2014).https://doi.org/10.1038/nchem.1891
More about the authors
Johanna L. Miller, jmiller@aip.org




