A mysterious player on the atmospheric stage
DOI: 10.1063/PT.3.1739
There’s not much sulfuric acid in the atmosphere—its concentration is measured in parts per trillion—but it makes its presence known by condensing into aerosol particles. Near Earth’s surface, the aerosols pose a hazard to human health; at higher altitudes, they attract water to form clouds, which affect both weather and climate. Atmospheric sulfuric acid forms when SO2 is oxidized into SO3, which combines with water to make H2SO4. The oxidation step is attributed to a reaction with OH radicals, highly reactive short-lived molecules that are formed by sunlight.
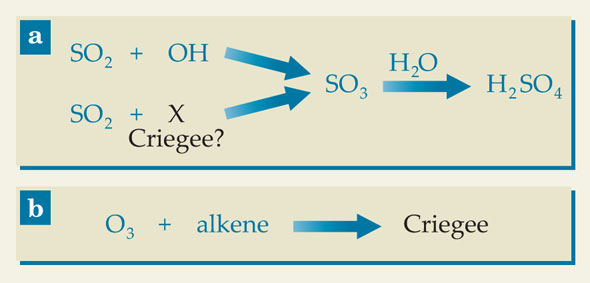
Now Lee Mauldin III (University of Helsinki and University of Colorado Boulder) and an international team of colleagues have shown 1 that there must be another atmospheric oxidant that, in some environments at least, can rival OH in its capacity for turning SO2 into H2SO4. The competing reactions are shown in panel a of the figure. The researchers have built up a wealth of indirect evidence that the new oxidant is a Criegee intermediate, a type of unstable molecule that forms in a reaction between ozone and alkenes (unsaturated hydrocarbons), as shown in panel b. But in the absence of a direct measurement, they identify it only as “X.”

The conversion (a) of sulfur dioxide to sulfuric acid is largely attributed to OH, which reacts with SO2 in the presence of oxygen to form SO3. Lee Mauldin and colleagues have shown that there must be another molecule, which they identify as X and suggest may be a Criegee intermediate, that does the same job. (b) Atmospheric Criegee intermediates form in a reaction between ozone and alkenes (unsaturated hydrocarbons).
The discovery was a product of work to measure atmospheric OH concentrations. A long-outstanding experimental challenge of atmospheric chemistry, OH measurements have been performed by only a handful of teams worldwide, including Mauldin’s. The typical method is to combine an atmospheric sample with enough SO2 that all the OH present reacts and forms H2SO4.
To account for the possibility of other sources of H2SO4, researchers repeat the measurement while adding to the sample not just SO2 but also a scavenger compound that destroys OH radicals more quickly than they can react with SO2. The difference in H2SO4 concentrations formed with and without the scavenger thus gives the OH concentration. As Mauldin recounts, “At first, we were more interested in getting the OH measurement right” than in uncovering the source of the background H2SO4 that formed in the presence of the OH scavenger. But as the OH data accumulated, it soon became clear that the background was often too strong to ignore.
The background exhibited some patterns. It correlated with ozone concentrations. And it was especially high in forested areas, where certain alkenes give evergreen trees their characteristic scents. Mauldin and colleagues took to the laboratory to check the correlations more precisely and found that they held: Add the necessary ingredients for a Criegee intermediate, and you oxidize more SO2.
That’s strong evidence, but not proof, that the new oxidant X is a Criegee intermediate. Observing X directly, rather than via its reaction products, could close the case, but that would be a difficult measurement: Only in the past year was a gas-phase Criegee intermediate directly observed for the first time (see Physics Today, March 2012, page 17
References
1. R. L. Mauldin III et al., Nature 488, 193 (2012).https://doi.org/10.1038/nature11278
More about the authors
Johanna L. Miller, jmiller@aip.org




