Ultrasound-mediated drug delivery
DOI: 10.1063/PT.3.3106
To many, the term medical ultrasound conjures snowy images of nascent life in the womb. Indeed, its relatively low cost, portability, and impeccable safety profile have propelled diagnostic ultrasound into becoming one of the most widespread imaging methods in the world. So it may seem somewhat contradictory that ultrasound can also induce a broad spectrum of bioeffects in tissue.
Ultimately, ultrasound is a form of energy. It interacts with the body’s tissues during propagation, and in the process it can deposit heat, displace tissues, and initiate cavitation events in which gas bubbles form and violently oscillate or collapse. Those interactions can in turn elicit bioeffects, albeit at exposure levels that are typically orders of magnitude higher than those used for imaging purposes.
From the inception of the field of biomedical ultrasound in the mid 20th century, researchers have appreciated the potential implications of ultrasound-induced bioeffects—both from the perspective of designing imaging systems to avoid tissue damage and from the perspective of harnessing those effects for therapeutic purposes. In the ensuing decades, foundational research advanced the understanding of ultrasound as a source of energy that could destroy target tissues in the body. (For more on the early history of medical ultrasound, see the article by Bill O’Brien and Floyd Dunn, Physics Today, October 2015, page 40
The noninvasive nature of therapeutic ultrasound made it an enticing concept—surgery without a scalpel—but in practice it failed to gain widespread traction in the clinic. To a large degree, the failure can be attributed to early technical limitations. Doctors could not effectively expose relevant tissue volumes and locations to ultrasound beams in a timely and controlled manner.
Over the past two decades, however, researchers have dramatically improved their ability to perform controlled treatments. Advancements have come in transducer technology to deposit energy within spatially localized target regions and in imaging-guidance tools to monitor thermal and cavitation activity. Those advances have led to significant inroads in thermal therapy for clinical applications, such as cancer treatment. (See the article by Gail ter Haar, Physics Today, December 2001, page 29
How to make a magic bullet
Be they drugs, stem cells, or DNA, therapeutic agents generally target specific regions of the body—for example, tumors or diseased tissue in an organ. As Nobel Prize–winning immunologist Paul Ehrlich articulated more than a century ago, the ideal therapeutic agent should act like a magic bullet that kills only its intended target.
An enduring obstacle to the effectiveness of many drugs is that they don’t reach their intended target at sufficiently high concentrations. When given intravenously, they must travel intact through the circulatory system, and on reaching a tumor or other diseased tissue, they must exit the bloodstream through the walls of microvessels and circulate through the extravascular space. The half-lives of agents in the bloodstream are frequently on the order of only tens of minutes, and only a tiny fraction of the original amount actually reaches the target tissue.
Enormous efforts have been directed at developing methods that locally increase drug concentrations to therapeutically relevant levels in tissue. One approach is to use delivery vehicles to prolong drug lifetime in the body. A prime example is to encapsulate the drug inside a small spherical structure—typically less than 200 nm in size—called a liposome. Most often, the protective outer shell of a liposome is made from phospholipids, a main molecular ingredient in cell membranes. Such delivery vehicles can be designed to release their therapeutic payloads using physical means—for example, by regional heating in the case of thermosensitive liposomes.
The application of external energy to cause the local release and uptake of drugs is a powerful paradigm. It offers doctors the ability to increase the achievable dose at the target and simultaneously reduce side effects. The primary energy sources in current use are light, RF electrical currents, microwaves, and focused ultrasound. Ultrasound is particularly appealing because it can be configured to noninvasively access a wide range of sites in the body. In addition, ultrasound does more than simply trigger the local release of drugs. Through thermal, mechanical, and chemical mechanisms, it can induce therapeutically relevant bioeffects such as enhanced vascular permeability and motion in fluids and tissues.
In a biomedical context, ultrasound is an umbrella term that encompasses all high-frequency (typically 0.1–50 MHz) mechanical waves that propagate in the body. For compressional waves—the type most commonly exploited for imaging and therapeutic purposes—oscillating particle motions with amplitudes on the order of nanometers give rise to heat deposition. Along with scattering effects, those oscillations result in a loss of power during ultrasound propagation. Absorption varies between tissue types and increases with frequency. The elevation in temperature at a site of exposure is related to the time-averaged ultrasound intensity, after thermal diffusion and the convective heat sink associated with blood flow are accounted for.
Ultrasound thermal therapy exploits that heat deposition. Different therapies are generally divided into two categories: ablation or hyperthermia. Ablative ultrasound employs high-intensity pulses of short duration (typically less than 30 seconds) to rapidly induce thermal lesions in the focal zone. Hyperthermia applies lower-intensity ultrasound for longer durations (typically 20 minutes or longer) to elevate temperatures to 40–45 °C. Since the 1980s researchers have gathered extensive evidence that radiotherapy can be enhanced by combining it with hyperthermia. 1 Unfortunately, the lack of effective methods to monitor and control temperature has hindered widespread clinical adoption.
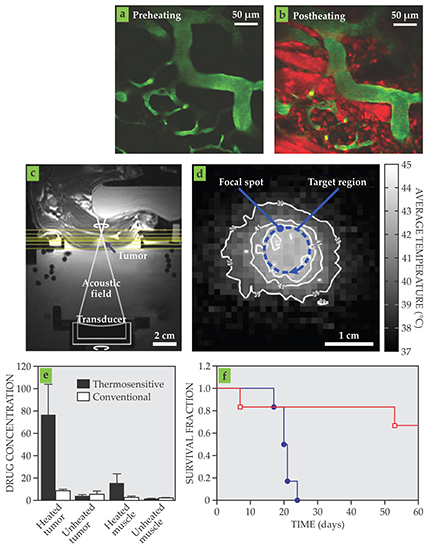
More recent investigations that pair ultrasound-induced hyperthermia with thermosensitive liposomal formulations of chemotherapy drugs have achieved potent preclinical results. 2 With image-guided temperature control, the approach can increase drug concentrations in a tumor 10- to 20-fold relative to using a conventional drug formulation (see figure 1). The magnitude of the delivery enhancement has the potential to fundamentally affect the treatment of many tumor types. Ultrasound-induced hyperthermia not only results in the targeted release of drug payloads from the delivery vehicles in a tumor, but heating can also locally enhance vascular permeability and make tumor tissue more susceptible to chemotherapy drugs.

Figure 1. Therapeutic effects of hyperthermia-mediated chemotherapy. (a–b) Two-photon microscopy images of a tumor during treatment illustrate increased drug release when tissue is heated (hyperthermia) by focused ultrasound. The blood vessels are green and the released drug is red. (c–d) MRI effectively guides ultrasound hyperthermia. The tissue image (c) and MRI-derived temperature map (d) of a tumor in a rabbit thigh show controlled and targeted temperature patterns obtained with MRI-guided ultrasound. (e) Relative to when the drug is given in conventional form, drug concentrations in target tissue dramatically increase when a thermosensitive formulation is administered in combination with controlled heating. (f) The enhanced drug delivery when a thermosensitive drug formulation is combined with MRI-guided ultrasound (red) results in pronounced survival increases in mice compared with administering the drug alone (blue). (Images in panels a and b courtesy of Marc Santos. Panels c and d adapted from ref.
In addition to thermal effects, the nonlinear relationship between acoustic pressure and density causes a propagating ultrasound wave to impart momentum to tissue. Bulk tissue displacements in the focal region can readily be on the order of hundreds of microns. Researchers hypothesize that displacement-induced strain gradients lead to a “loosening” of the extravascular space. 3 The exploitation of such effects to facilitate drug delivery is an active area of investigation.
Blowing bubbles
Sufficiently high ultrasound intensities can induce cavitation. In pure liquids, vapor-filled cavities form if instantaneous local negative pressures exceed the tensile strength of the liquid. The cavitation threshold in water is tens of megapascals. 4 However, in soft tissues, the presence of microscopic gas bodies and other cavitation seeds lowers the threshold precipitously.
Cavitation can produce a range of therapeutically important effects. It is frequently divided into the stable regime with long-lived oscillating bubbles and the inertial regime with quickly collapsing bubbles. True to its name, the inertial regime is dominated by the inertia of the surrounding fluid during the bubble-collapse phase. The temperature within the bubble core can reach thousands of kelvin, light emission (sonoluminescence) is stimulated, and free-radical species are produced. Mechanical tissue damage is generally associated with inertial cavitation, and, in fact, it has been harnessed to fragment tissue into subcellular debris.
Although researchers have long recognized that vascular permeability is increased by cavitation induced by stand-alone ultrasound, the procedure has not been extensively investigated as a means of enhancing drug potency. An exception is sonodynamic therapy, which uses cavitation in conjunction with photosensitizers to promote tissue death. 5 The precise mechanisms of sonodynamic therapy have yet to be fully established, but the leading hypothesis is that the generation of free-radical species and the activation of photothermal effects via sonoluminescence are the primary factors involved.
A revolution in the use of cavitation for therapeutic ultrasound occurred with the help of microbubble contrast agents. 6 Originally developed in the 1980s and 1990s for imaging blood flow, microbubbles encapsulate high-molecular-weight gas cores inside thin, compliant biocompatible shells. A typical example is a phospholipid-monolayer shell filled with perfluorocarbons, biologically inert compounds made of fluorine and carbon. Microbubble formulations contain a range of bubble sizes, generally 1–8 μm in diameter. The size range ensures that bubbles are large enough to be constrained to remain within blood vessels and that subpopulations of bubbles are resonant in the 1–10 MHz frequency range relevant to both diagnostic and therapeutic ultrasound.
When stimulated by ultrasound waves, microbubbles can undergo substantial oscillations at relatively low pressures. Under resonance conditions, bubble-surface oscillation amplitudes can reach some micrometers. Inside microvessels that are less than 50 µm in diameter, those oscillations can deform microvascular walls and induce microscale fluid flow in the blood (see figures 2a and 2b). Collapsing microbubbles can even produce needle-like microjets directed toward vascular walls.

Figure 2. Focused ultrasound and microbubbles. (a–b) Ultrasound waves (curved lines) cause microbubbles (large spheroids) to oscillate inside blood microvessels. The resulting fluid flow and vascular wall deformations increase microvascular leakiness and promote the transport of therapeutic agents (small spheres) from the microvessel into the extravascular space, as indicated by the blue arrows. (c–d) Two-photon microscopy images of brain microvasculature before (c) and after (d) ultrasound exposure. The green arrows point to areas where fluorescent dye, normally constrained to stay in the microvessels, has leaked out. (Images courtesy of Charissa Poon.)
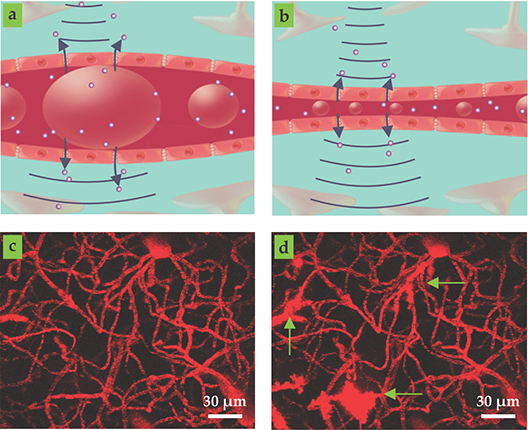
For diagnostic imaging, exposure conditions must be limited to avoid side effects. In the late 1990s, however, researchers realized that microbubble oscillations induced by relatively low-intensity ultrasound produced vascular bioeffects that promote the transport of drugs across microvascular walls into the extravascular space. 7 A comprehensive mechanistic framework for the phenomenon has yet to be established. However, a likely explanation is that the expansion and contraction of the bubbles produces stresses at the vessel walls that promote the passage of drugs out of the blood, either through or between endothelial cells, the innermost lining of the vessel walls. Mechanical stimulation has been shown to create pores in the cell membranes and can also activate mechanosensitive ion channels, protein tunnels in cell membranes that allow ions to move in and out of cells. The activation of those ion channels in turn triggers a cascade of intracellular processes that actively transport the drug molecules through the cells. 8
A wealth of data have now accumulated on ultrasound-enhanced drug transport through microvascular walls in a range of tissue types and with various therapeutic agents. The most widely studied site is the brain, which contains specialized microvessels that present a particularly onerous barrier to the penetration of drugs. The conditions under which the barrier, known as the blood–brain barrier, can be prodded into being temporarily leaky (see figures 2c and 2d) are now relatively well established. 8 That information provides an important rational basis for treatment design: Pressures must be high enough to stimulate substantial, stable microbubble oscillations but low enough to avoid tissue damage. In other tissue types, such as tumors, evidence suggests that inertial cavitation may be preferable for promoting drug delivery.
Considerable work remains to fully understand and control cavitation under different circumstances. Exposure pressures have varied widely between studies. Nonetheless, the time-averaged intensities are generally far lower than for thermal therapies, and macroscopic temperature elevations are not required to achieve the effects.
Acoustic delivery
The list of therapeutic agents that have been assessed is not limited to conventional drugs such as chemotherapeutics. It also includes enzymes, genetic material, and even stem cells and immune cells. 8 , 9 The large majority of studies have used intravenous co-injection of therapeutic agents and microbubbles that circulate freely through the body but are exposed to ultrasound only locally, at the target site. Because both the microbubbles and the drugs are clinically available, that approach presents a faster route to clinical approval. However, the therapeutic agents still circulate in active form throughout the body: Most not only are wasted but could induce side effects.
Those considerations have prompted the development of delivery vehicles that can be activated acoustically. In fact, efforts to make such vehicles preceded those related to permeability enhancement. 9 One incarnation involves imbedding therapeutic agents into the microbubble’s shell or attaching them on the outside, where they remain bound until they are released by ultrasound. Other approaches include filling the microbubbles with bioactive gases such as nitric oxide or oxygen or adhering a layer of smaller liposomal drugs to bubble surfaces. Liquid nanodroplets (commonly superheated perfluorocarbons) are also potential drug-delivery vehicles. Loaded with drugs, the nanodroplets can circulate intact in the blood until they undergo acoustically triggered phase changes.
An entirely different drug-delivery route is to employ ultrasound to promote the transdermal delivery of therapeutic agents—insulin, for example—into the bloodstream. In that approach, cavitational or thermal mechanisms enhance drug penetration through the skin’s surface. 10
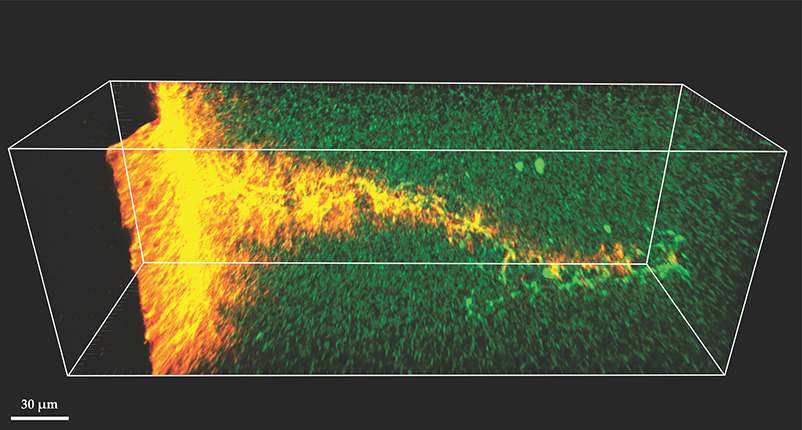
The approaches described above aim to deliver drugs or other therapeutic agents into the extravascular space. But one of the earliest combinations of ultrasound and therapeutic agents, dating back to the 1970s, was to break up blood clots in blood vessels, a technique now known as sonothrombolysis. 11 Figure 3 shows an example in which microbubbles degrade the protein matrix of a clot and promote fluid uptake.

Figure 3. Two-photon microscopy image of a fibrin-protein clot into which a microbubble has entered under the influence of ultrasound stimulation. The penetrating microbubble disrupts the protein network structure (green) that holds the blood clot together. Fluid flow deep into the clot along the microbubble’s path is revealed by fluorescent nanobeads (orange). (Image courtesy of Christopher Acconcia.)
Separate from facilitating the delivery of therapeutic agents, ultrasound-based hyperthermia and cavitation can induce a host of therapeutic bioeffects. Among the effects are inflammation, the triggering of an immune response, and vascular damage. Although those effects are not directly related to drug delivery, they may favorably impact the effects of drugs.
Control and conquer
Various ultrasound-based effects enhance the local delivery and activity of therapeutic agents. To exploit those effects in the clinic, one must be able to deliver ultrasound energy in a controlled manner to targeted regions, and the delivery of that energy and its resulting effect must be monitored. Advances in those areas, which involve the development of image-guided ultrasound systems, specialized transducers, and the linking of signals to bioeffects for specific applications and sites, are driving the field forward at a rapid pace.
At the heart of any ultrasound system is a transducer that converts electrical energy into mechanical energy and directs it into specific regions of the body. Early ablative ultrasound systems used high-gain curved apertures or lenses to geometrically focus acoustic energy into target regions the size of rice grains. To expose larger volumes, clinicians translated the focus through a sequence of discrete locations—an approach that was prohibitively long for many situations.
The development of array-based transducer technology has resulted in greatly improved flexibility in the deposition of ultrasound energy. Introduced in the 1970s, arrays partition the transducer aperture into smaller, independently controlled elements. Electrically stimulating the individual elements with appropriate time delays yields a beam that can be rapidly focused and steered over relatively large tissue volumes. That approach was first implemented for diagnostic ultrasound imaging, and although the basic physics is the same for therapeutic arrays, the two applications can have distinctly different design considerations.
The requirements of therapeutic arrays have spurred innovations in methods that correct for intervening bone between the ultrasound source and the target—for example, the brain or the liver. Disparities between the propagation velocity in bone and surrounding tissue, along with shape and thickness variations, give rise to phase differences between different wave paths that act to displace and distort the focal region. Compensation methods apply appropriate phase corrections, transducer element by transducer element. One approach uses corrections based on computed tomography x-ray imaging to estimate bone density, which is inversely related to the speed of sound. 8
The two primary approaches for guiding the focal region of the therapeutic beam in tissue are ultrasound imaging and magnetic resonance imaging. For ultrasound-guided systems, an imaging transducer is placed in a therapeutic array and the operator selects a region of interest based on the ultrasound images. 12 Ultrasound imaging can detect ablated tissue, but temperature monitoring for hyperthermia applications still presents a challenge. Various techniques, such as those based on echo arrival-time shifts that arise from temperature-dependent changes in propagation velocity or volumetric expansion, have been developed that allow the operator to infer temperature changes with ultrasound.
An integrated ultrasound imaging transducer also provides a means to monitor and exploit cavitation signals derived from the therapeutic pulses. In conventional diagnostic microbubble imaging, short pulses about 1 µs long stimulate nonlinear emissions that are subsequently used to create high-resolution spatial maps of the bubbles. Therapeutic pulses, however, tend to be longer, so a different method is necessary. The most common one is to detect signals within specific frequency ranges, such as subharmonics, second harmonics, or a bandwidth associated with inertial cavitation. The most successful methods to date employ passive acoustic mapping, adapted from geophysical imaging. (See the article by Roel Snieder and Kees Wapenaar, Physics Today, September 2010, page 44
A pivotal change in the trajectory of therapeutic ultrasound occurred when researchers began to incorporate high-intensity focused ultrasound (FUS) systems into MRI scanners in the 1990s. 2 , 13 The advantages of MRI include excellent soft-tissue contrast, high resolution, and three-dimensional imaging capability appropriate for guidance. In addition, it has the capacity to assess thermal dose, the time-integrated temperature history that is key to causing tissue damage. The ability to monitor temperature and therefore guide thermal therapies in a noninvasive manner has obviated the need for invasive thermocouples (see figures 1c and 1d). The approach has been successfully tested in a range of tissue types, though its performance degrades in fatty tissues and in the presence of tissue motion. Although current MRI-guided high-intensity FUS systems are not optimized for hyperthermia treatments, the approach is well suited for use in delivering thermosensitive drugs. An example transcranial ultrasound configuration is shown in figure 4.

Figure 4. MRI-guided transcranial ultrasound. (a) The photograph shows an MRI-based transcranial ultrasound system. (b) The schematic illustrates a clinical system in which a computer-controlled hemispherical transducer array, guided by MRI, delivers ultrasound to a targeted region in the brain. (c) This MRI image shows a rabbit brain after the blood–brain barrier has been opened by ultrasound and microbubbles. The white region near the center of the image shows where MRI contrast agents have leaked out of blood vessels and indicates the successful opening of the barrier.
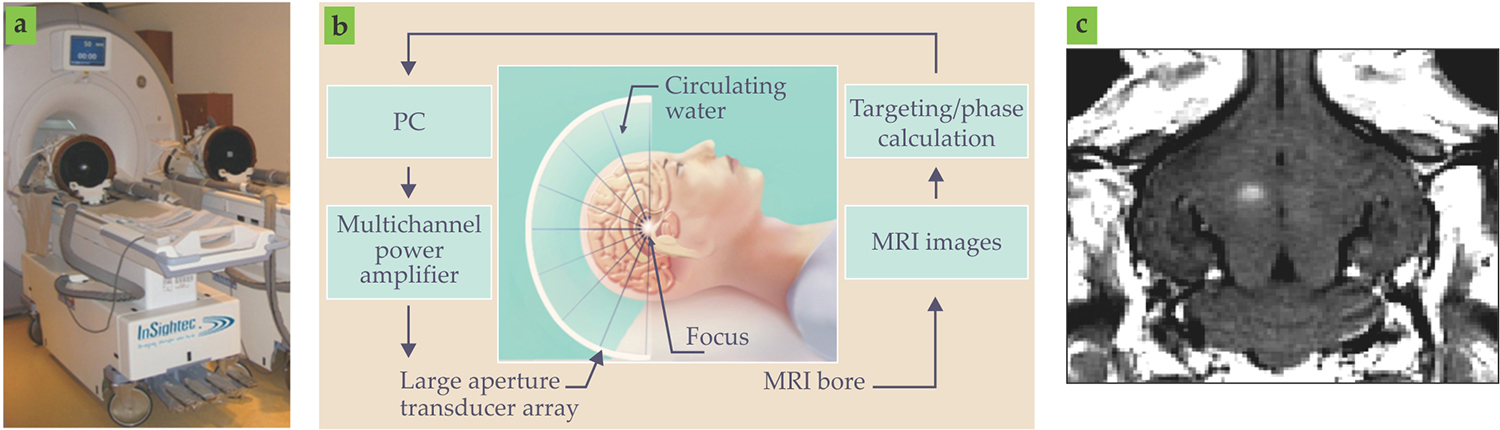
Numerous preclinical studies on animal models have investigated microbubble-mediated therapies in an MRI environment. In most of those studies, cavitation was either monitored with a single cavitation detector or not monitored at all. However, early tests of cavitation-feedback control schemes show considerable promise to enable more robust and safe delivery performance in the brain. 8 In addition, when MRI contrast agents, constrained to remain within the vasculature under normal conditions, leak out posttreatment, they can be used as an indicator of treatment success (see figure 4c). Together with microbubble imaging, cavitation mapping capabilities will soon permit more sophisticated control and treatment schemes. 14
Collectively, advances in transducer technology and beam-guidance systems have paved the way for a broad array of applications that use a host of therapeutic agents. Specific instrumentation and approaches are being developed for a range of sites within the body.
Local delivery for global impact
Arguably one of the applications closest to clinical adoption is sonothrombolysis. 11 An important sonothrombolysis milestone came in 2001 when researchers discovered that pulsed-wave Doppler ultrasound—a tool originally designed to assess blood flow in cerebral vessels—enhanced the effects of drugs for breaking up blood clots in stroke patients. Due to the poor performance of current blood-clot treatments, significant efforts, including clinical trials, are under way to develop sonothrombolysis applications for the brain, heart, and legs. Recent developments in transcranial ultrasound delivery could significantly improve treatments relative to existing methods.
Indeed, such technical developments promise to improve treatments for a spectrum of central nervous system disorders. 8 The advances are spurred on by accumulating clinical experience using commercially available transcranial systems to treat brain tissue thermally, and by emerging evidence from nonhuman-primate studies that show microbubbles can safely and transiently open the blood–brain barrier. Notably, the first human trials that use microbubbles to enhance chemotherapy delivery for brain cancer are under way at the Sunnybrook Research Institute. Experience gained in that and other upcoming clinical trials will be central to advancing and refining methods that can be employed in a range of applications.
A prime candidate for ultrasound-based treatment is Alzheimer’s disease. A body of preclinical evidence from animal studies indicates that ultrasound can improve the delivery of therapeutic antibodies and thereby reduce the burden of Alzheimer’s plaques. Interestingly, stand-alone ultrasound treatments have been reported to reduce plaque load and enhance cognition and memory in transgenic mice. Other promising applications include the delivery of therapeutic agents to promote recovery from stroke and treatments of Parkinson’s and Huntington’s diseases.
The treatment of a wide range of cancers stands to benefit from FUS-mediated drug delivery. 2 , 5 , 8 , 15 Devices configured to perform ablative therapy in brain tumors and other sites such as the breast, liver, and prostate are already commercially available. Pending the development of more optimized approaches, the devices will permit investigations of hyperthermia-mediated thermosensitive chemotherapy drug release.
In addition to thermal therapy, a rapidly accumulating body of work has demonstrated the therapeutic effects of FUS and microbubbles coupled with anticancer agents. An initial clinical feasibility study has been reported for pancreatic cancer. 15 Fortuitously, a paradigm shift is occurring in cancer therapy. Many doctors and researchers now believe that the localized treatment of several primary or metastatic tumors can have a more widespread therapeutic effect on the entire tumor burden.
Cardiovascular applications are not limited to the use of sonothrombolysis. A heart attack can cause sustained reduction of blood flow to the heart itself and result in what is called ischemic injury. There is a pressing need for treatments that promote the recovery of such tissue. Animal studies have shown that the use of FUS to promote gene therapy that stimulates new vessel growth in injured heart tissue increases blood flow and heart function. 9 , 16 Efforts are also under way to promote drug delivery into atherosclerotic plaques, the origin of most heart attacks.
Outlook
Ultrasound-mediated drug delivery has enormous potential to treat a broad range of diseases and conditions. From a clinical perspective, it is still at an embryonic stage, and its ultimate impact on health care remains to be seen. However, two significant factors are driving advances in ultrasound-based therapies toward clinical adoption: It may be capable of applying treatments that are poorly addressed with current approaches and do so in an economically viable manner. The keys will be to improve our understanding of therapeutic processes and to develop treatment systems and approaches tailored to specific applications. Both will entail inherently interdisciplinary efforts at the interface of physics, engineering, biology, and clinical medicine.
References
1. G. ter Haar, C. Coussios, Int. J. Hyperthermia 23, 89 (2007). https://doi.org/10.1080/02656730601186138
2. N. Hijnen, S. Langereis, H. Grüll, Adv. Drug Deliv. Rev. 72, 65 (2014). https://doi.org/10.1016/j.addr.2014.01.006
3. S. Dromi et al., Clin. Cancer Res. 13, 2722 (2007). https://doi.org/10.1158/1078-0432.CCR-06-2443
4. T. G. Leighton, The Acoustic Bubble, Academic Press, 1994).
5. A. K. W. Wood, C. M. Sehgal, Ultrasound Med. Biol. 41, 905 (2015). https://doi.org/10.1016/j.ultrasmedbio.2014.11.019
6. D. L. Miller, Prog. Biophys. Mol. Biol. 93, 314 (2007). https://doi.org/10.1016/j.pbiomolbio.2006.07.027
7. D. L. Miller et al., J. Ultrasound Med. 27, 611 (2001); https://doi.org/10.1016/S0301-5629(01)00343-X
R. J. Price et al., Circulation 98, 1264 (1998). https://doi.org/10.1161/01.CIR.98.13.12648. A. Burgess et al., Expert Rev. Neurother. 15, 477 (2015); https://doi.org/10.1586/14737175.2015.1028369
M. Aryal et al., Adv. Drug Deliv. Rev. 72, 94 (2014); https://doi.org/10.1016/j.addr.2014.01.008
E. E. Konofagou et al., Curr. Pharm. Biotech. 13, 1332 (2012). https://doi.org/10.2174/1389201128006243649. E. C. Unger et al., Adv. Drug Deliv. Rev. 56, 1291 (2004). https://doi.org/10.1016/j.addr.2003.12.006
10. A. Azagury et al., Adv. Drug Deliv. Rev. 72, 127 (2014). https://doi.org/10.1016/j.addr.2014.01.007
11. K. B. Bader, G. Bouchoux, C. K. Holland, in Therapeutic Ultrasound, J.-M. Escoffre, A. Bouakaz, eds., Springer, 2016), p. 339.
12. E. S. Ebbini, G. ter Haar, Int. J. Hyperthermia 31, 77 (2015). https://doi.org/10.3109/02656736.2014.995238
13. K. Hynynen, J. Magn. Reson. Imaging 34, 482 (2011); https://doi.org/10.1002/jmri.22649
N. McDannold, Int. J. Hyperthermia 21, 533 (2005). https://doi.org/10.1080/0265673050009607314. M. A. O’Reilly, K. Hynynen, Radiology 263, 96 (2012). https://doi.org/10.1148/radiol.11111417
15. S. Kotopoulis et al., Med. Phys. 40, 072902 (2013). https://doi.org/10.1118/1.4808149
16. E. C. Unger et al., Adv. Drug Deliv. Rev. 72, 110 (2014). https://doi.org/10.1016/j.addr.2014.01.012
17. R. M. Staruch et al., Int. J. Hyperthermia 28, 776 (2012). https://doi.org/10.3109/02656736.2012.736670
18. R. M. Staruch, K. Hynynen, R. Chopra, Int. J. Hyperthermia 31, 118 (2015).https://doi.org/10.3109/02656736.2014.992483
More about the authors
David Goertz and Kullervo Hynynen are biomedical physics professors at the University of Toronto and senior scientists at the Sunnybrook Research Institute in Toronto.








