Treating Cancer with Protons
DOI: 10.1063/1.1522215
In the treatment of cancer, an ideal radiation source would deliver a near-uniform dose to the target and nothing outside it. This paragon is unachievable. The next best thing would be a source that delivers most of the dose within the target volume and relatively little outside it. In a seminal 1946 article, 1 Robert Wilson recognized that charged particles such as protons come close to accomplishing this highly desirable goal. Spurred by his vision, researchers at a number of centers throughout the world have been evaluating therapies based on protons and other light ions for the past four decades. 2,3
The research has largely been done in physics laboratories, where the necessary accelerators are already in place. Only recently, prompted by what appear to be very positive clinical results, have protons moved closer to mainstream radiation oncology. A number of hospital-based proton medical facilities are now in use or under construction in several countries. The task now is to ensure that protons attain their utmost potential and that their clinical value be critically assessed.
To appreciate the particular benefits of proton therapy, it is essential to understand how protons interact with matter. As they pass through tissue, protons lose energy—and hence deposit dose—gradually. Electromagnetic interactions with orbiting electrons constitute the main energy loss mechanism. The protons’ rate of energy loss is proportional to the inverse square of their velocity, and the secondary electrons, being short-ranged, give up the energy they gained from the protons quite locally. Consequently, the local deposition of energy rises sharply as the protons slow down—that is, as they penetrate farther into the medium. Range straggling—statistical fluctuations in energy loss which result in fluctuations of about 1% of range—somewhat blur the energy loss distribution, which adopts the characteristic Bragg peak (see figure 2 on page 36 of the introductory article).

The Northeast Proton Therapy Center at Massachusetts General Hospital in Boston. The floor plan illustrates the scope and complexity of an operational proton medical facility.
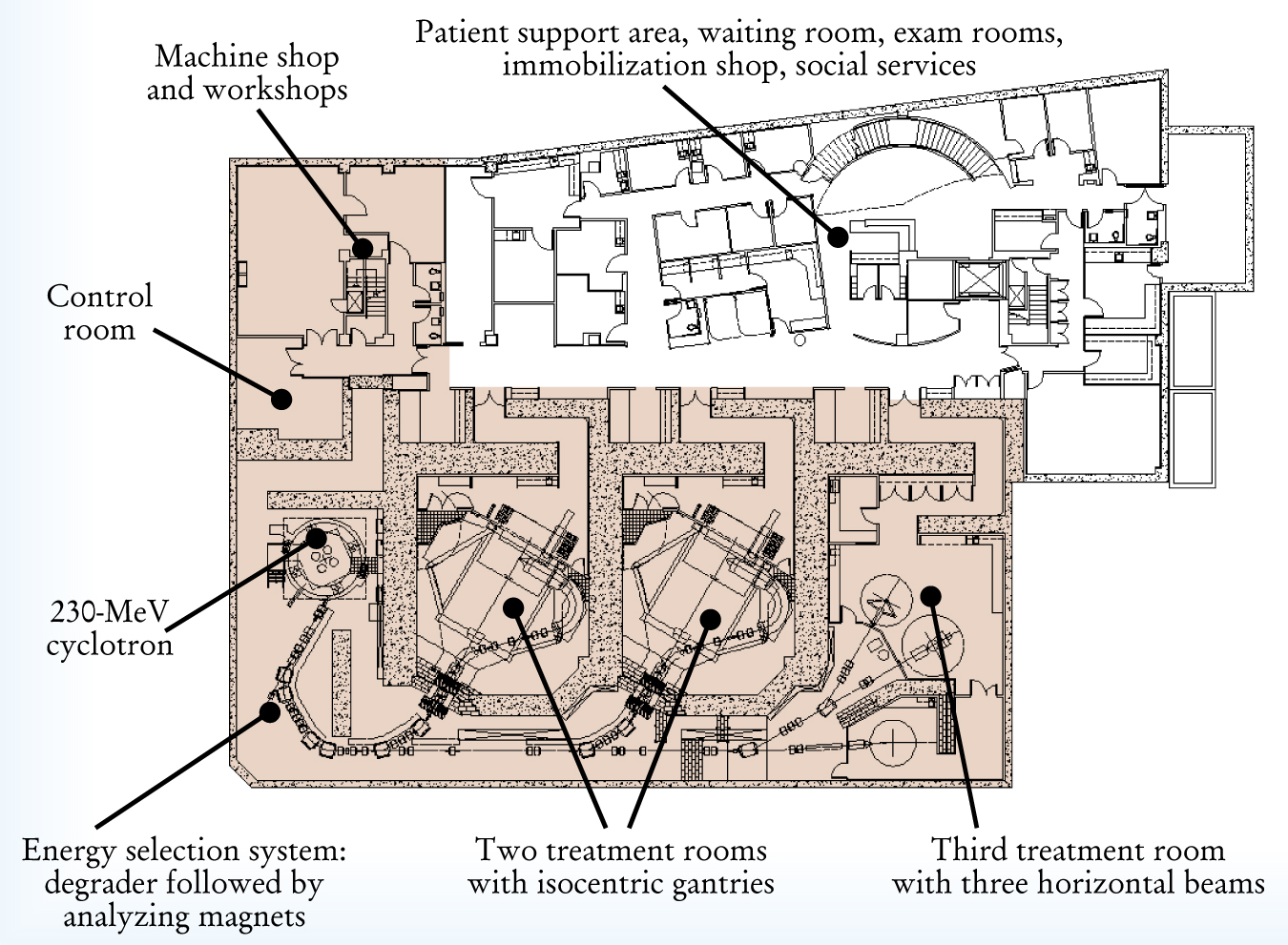
Protons also interact with nuclei. Nuclear collisions gradually reduce the fluence of primary protons in the beam, while the nuclear fragments broken off by the protons deposit additional dose locally. Another important interaction is multiple Coulomb scattering, which spreads the beam out laterally. Through Coulomb scattering, an initially infinitesimally narrow beam will acquire a nearly Gaussian lateral distribution with a full width at half maximum approximately 5% of the range.
These atomic and nuclear interactions have three inescapable consequences for proton therapy. First, the dose of a monoenergetic proton beam diminishes sharply downstream of the Bragg peak, typically dropping from 80% to 20% of the peak dose in a few millimeters. Second, multiple scattering in the patient dominates how the dose falls off laterally. The resultant penumbra, from the clinical point of view, is excellent for low energy (<100 MeV) protons; very good for medium energy protons (100–150 MeV), for which the dose typically falls from 80% to 20% in about 6 mm; and a bit larger than one would really like for protons of higher energy. Third, beam penetration within the patient can be easily controlled, either by adjusting the beam energy or by interposing material upstream.
Designing doses
The composition and density of patients’ tissues are highly inhomogeneous. Because the inhomogeneities affect dose distribution, proton beams must be designed to account for the patient’s anatomy. To this end, one needs a map of the patient’s tissues. Fortunately, CT provides just such a map. Because they are derived from x-ray transmission measurements, CT scans are in units of relative x-ray absorption coefficients. However, quite satisfactory transformations to proton-stopping powers (relative to water) can be easily derived from the CT data. The spatial resolution needed for such maps is set by the scale of multiple Coulomb scattering (from one to a few millimeters). Happily, the resolution of CT data is a good match.
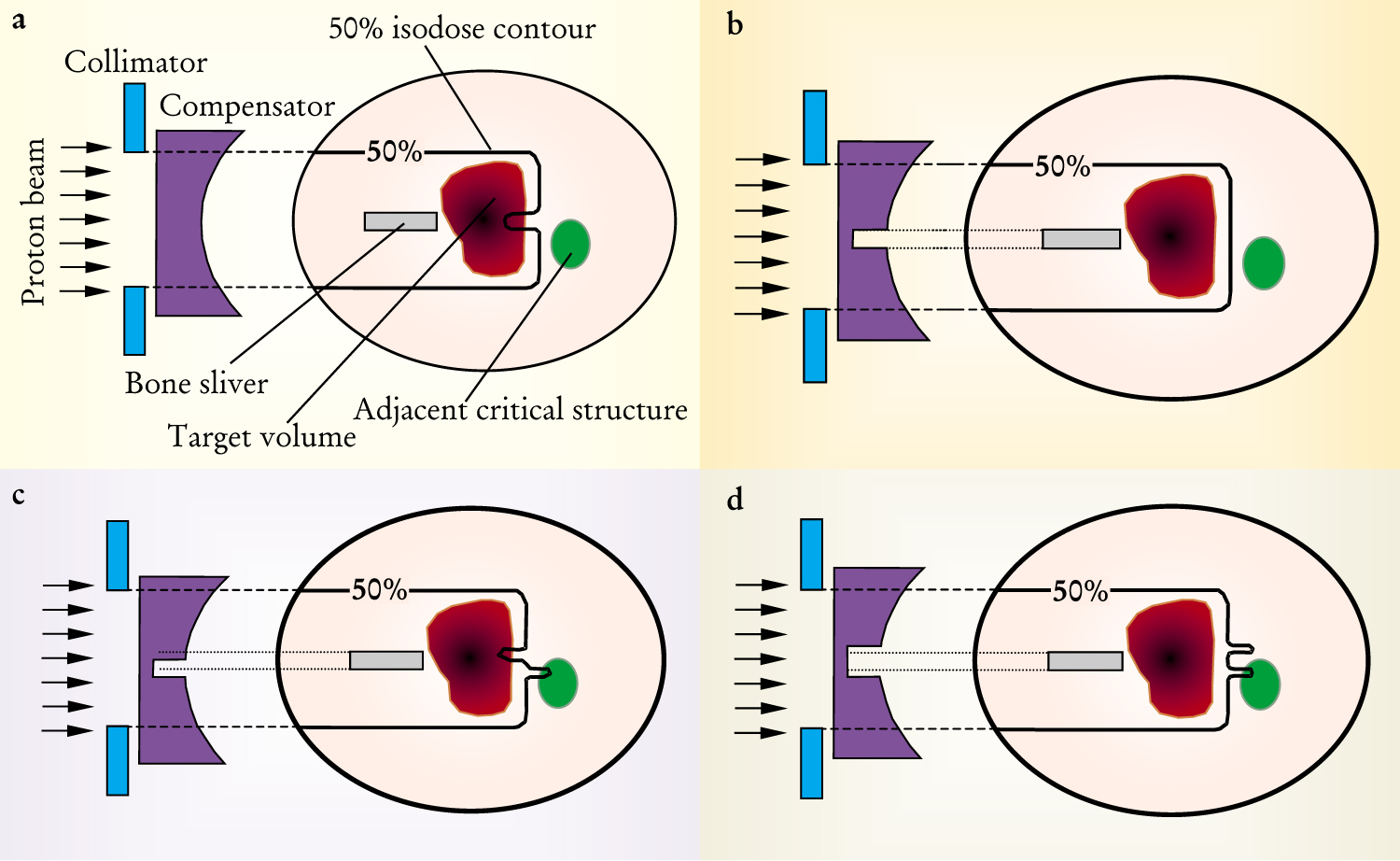
One might think that the ideal way to deal with an internal inhomogeneity would be to modify the energy of the incident beam where it shadows the inhomogeneity. However, because of multiple Coulomb scattering and the motion of the patient’s body and target organ, one cannot fully compensate for an internal inhomogeneity. As figure 1 illustrates, one way to deal with this limitation is to adjust the upstream compensation. If, for example, one adopts the strategy of complete tumor coverage (at the expense of over-irradiating healthy tissue), the upstream compensator can be widened by an amount determined by the combined extents of multiple scattering and motion.

Intervening inhomogeneities must be compensated for to create the right dose distribution. Here, compensation for a high-density bone sliver is achieved through a specially shaped material compensator. (a) No compensation is applied. As a result, part of the target does not receive the full dose. (b) First-order compensation is applied. Ideal target coverage appears to result. (c) Misaligned compensation is applied. The beam overshoots in some areas and undershoots in other areas. (d) “Opened” compensation is applied. Ideal target coverage is the result, but at the cost of overirradiating an uninvolved organ.
The estimation of the dose distribution of a proton beam has been approached in three ways. They are listed as follows in order of increasing accuracy.
4
Broad beam algorithms are based on measurements made in homogenous water phantoms. To compute beam penetration, the measurements are adjusted using calculations of the integrated effective densities along straight lines within the actual patient.
Pencil beam algorithms compute the dose as a superposition of pencil beams. These algorithms can take into account some aspects of differential scattering effects in laterally inhomogeneous materials.
Monte Carlo techniques can, in principle, incorporate all the essential physics of beam penetration, but are computationally intensive. To reduce computation times to practicable levels, algorithms with more limited physics have been developed.
Equipment and beam delivery
In part because the equipment is rather bulky, dedicated proton facilities have been installed in hospitals only recently. Protons with energies in the useful range of about 100 to 250 MeV have high magnetic rigidity and cannot be controlled without large, powerful magnets. For efficient beam use, one accelerator usually serves several treatment rooms, as illustrated in figure 2. The equipment should be conceived of as a push-button machine that does not require specialist operators.
The accelerator is the center of the facility, but, from the point of view of space, cost, and complexity, it represents only about 20% of the whole system. Both cyclotrons and synchrotrons have been used for proton beam therapy, and linear accelerators have also been considered. Driving the choice of accelerator are the general requirements of safety, reliability, and ease of operation and maintenance. The requirements of the beam-spreading technique are also important. High beam currents, however, are not required. Currents of tens of nanoamperes suffice.
Accelerator physics and related technologies have contributed enormously to the field of radiation therapy. There remain challenging problems. Some relate to the need to increase reliability while striving for simplicity and lower cost. Many problems remain in improving the overall system, and the accelerator in particular, for the purpose of beam scanning. New ideas include the use of superconductivity (warm or cold), slow extraction by stripping methods, variable energy cyclotrons, and a pulsed laser-driven proton source that could be compact—perhaps incorporated into the gantry head, reducing the cost and size of the system as a whole.
One of the most costly and technically complex components of a proton therapy facility is the mechanism for rotating the beam around the patient. One of two main approaches is adopted: a large-throw gantry with a diameter of 10–12 m or a compact gantry with a diameter of 4–7 m, as shown in figure 3. The choice of approach depends on the space available and on the method of beam delivery. 5 Because tumors frequently abut, or are very close to, critical healthy tissues, an overall beam-pointing accuracy of about 0.5 mm is needed. The positions of the gantry and patient must be extremely reproducible and, in the case of the patient, tightly controlled. Achieving this performance is a major technical challenge. A moving system that weighs about 100 tons must be controlled with submillimeter mechanical precision. And the shape invariance and positional stability of the beam must be guaranteed within a few tenths of a millimeter as the gantry rotates.

The compact gantry developed at the Paul Scherrer Institute in Villigen, Switzerland. At four meters, the diameter of the rotating part of the gantry is remarkably small compared with other machines. Two features of the design make the reduction in size possible. First, the beam scanning is performed before the beam is bent toward the patient. Second, the patient table is mounted eccentrically on the front of the gantry. The main degrees of freedom are: (1) the three scanning directions, one magnetically controlled, one mechanically controlled, and the third (in depth) implemented by interposing material mechanically into the beam; (2) rotation (α) of the beam around the patient (compensated by an equal and opposite ϕ rotation to keep the patient couch horizontal); and (3) rotation of the patient around a vertical axis (β) to allow noncoplanar beam directions.
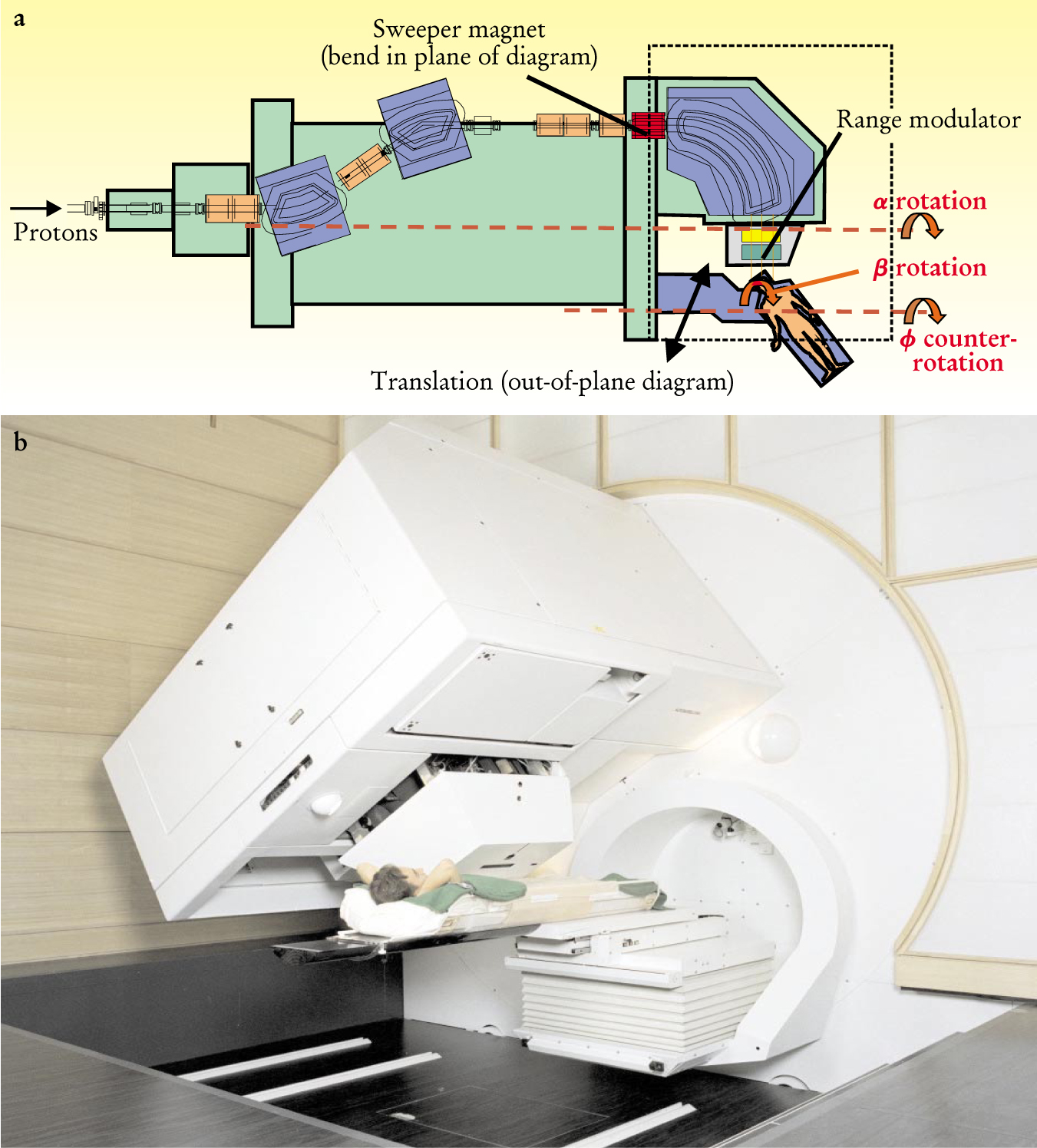
Target volumes typically range in size from a few milliliters to several liters, but the beam that emanates from the accelerator is extremely narrow and typically deposits 80% of its energy in a Bragg peak only six millimeters deep. As a consequence, the beam generally needs to be spread out in both width and depth. There are two main approaches for accomplishing this: passive scattering and active scanning.
Passive scattering is the more traditional technique. The box on page 49 shows some of the basic operating principles. To widen the beam, scattering material is interposed in such a way as to produce a homogeneous flux of particles throughout the solid angle covering the tumor. A sharp boundary to the dose distribution in the lateral direction is achieved using individually shaped collimators. Rotating range shifter wheels (or ridge filters) modulate the range. Depending on the size and location of the tumor, one selects elements from an arsenal of prefabricated scatterers and range shifter wheels. In most modern facilities, the scatterers and wheels are motorized on the gantry for automated insertion into the beam. Ensuring that the distal edge of the proton field coincides precisely with the posterior surface of the target volume is done through the use of patient-specific range compensators. These devices are thin when a large beam penetration is desired and thick when the target is closer to where the beam enters the patient.
Passive scattering has the limitation that the dose distributions are inherently uniform. Tailoring a distribution to cover the target necessarily involves trimming the proton field. A more versatile method for spreading the beam is known as active scanning. In this approach, also shown in the box on page 49, both the energy (hence, depth) and direction (hence, lateral position) of a pencil beam of protons are dynamically varied to fill the target volume step by step in an optimal pattern. Any physically possible dose distribution can be delivered, protons are not wasted, and no patient-specific hardware is required. Performing beam scanning safely and accurately does, however, require sophisticated control-system technology. Now that the technology is available, active scanning will achieve one of its major potentials: the implementation of intensity-modulated radiation therapy with protons.
Active scanning can be accomplished by magnetic or mechanical means, or by a combination of the two. Currently, only one active scanning system for protons is operational in a clinical setting: the compact spot-scanning gantry (figure 3) at the Paul Scherrer Institute in Villigen, Switzerland. At PSI, the beam is spread longitudinally by magnetic deflection and laterally by moving the patient table. To modulate the beam range, the amount of material in front of the patient is dynamically changed (typically in 50 ms). A similar scanning system for heavy-ion therapy has been developed at the Laboratory for Heavy-Ion Research (GSI) in Darmstadt, Germany. The GSI system features magnetic scanning in two lateral directions and dynamic variation of the beam energy.
The typical pencil beam size chosen for treating deep-seated tumors is about 5–8 mm full width at half maximum. Smaller beams are difficult to obtain because of multiple scattering in the equipment and in the patient. In practice, one has to cope with practical limitations due to the granularity of the dose distribution and the speed and time needed for a full scan (typically 10 000 individual spots per liter have to be applied in a few minutes). Because the whole energy of the beam is concentrated in a spot, safety is a major concern. Active scanning requires redundant measuring systems, double independent computers, and redundant fast beam abort systems in case a component malfunctions.
Active scanning’s major weakness is its sensitivity to organ (and tumor) motion. Organ motion, which is primarily due to the patient’s respiration, can markedly affect the dose distribution because a given point can either move away from the beam when it should lie within it (resulting in a lower than desired dose) or linger within the beam as it moves (resulting in a dose higher than desired). For this reason, active scanning is presently used to treat only well-immobilized tumors located in the head and in the lower pelvis. An interesting method for attacking the problem of organ motion is to trigger the beam delivery within a given phase interval of the breathing cycle. This method is the specialty of the Japanese centers that work with charged particle therapy, but it has not yet been applied to a scanning system.
A more futuristic solution to the problem of organ motion is to apply position corrections to the beam steering during scanning so that the beam follows the displacement of the tumor in real-time. Positional information could be measured, for example, by the tumor localization system developed by the TULOC group at PSI. In that system, a tiny magnetic coil is inserted through a biopsy needle into the body and anchored within a solid tumor. Its position is determined from the signals of several induction coils placed outside the patient. Further progress could be achieved with more advanced scanning methods in which the dose can be painted repeatedly without increasing the treatment time. This strategy could be carried out using dynamic control of intensity at the ion source to shape the dose distribution while the sweeper magnets run very fast.
Proton dose distributions
For a single field direction, passive scattering can provide excellent conformation of dose to the distal end of the target and good conformation laterally (figure 4(a)). However, because of the fixed depth-modulation of Bragg peaks across the whole field, passive scattering provides little conformation of dose to the proximal side of the target volume.

Calculated Dose distributions for irradiating a form of bone cancer called Ewing’s sarcoma. Each panel shows a single transverse section through the center of the tumor. The dose distribution is represented as semi-transparent color-wash (using the scale depicted at right, in which 100% indicates the desired dose to the tumor) overlaid on a CT section of the affected area. The target volume is delineated in yellow, and critical structures are outlined in red. Arrows indicate the incident field directions. (a) A single passively scattered proton beam (field). (b) A three-field approach in which all the fields are passively scattered. (c) A single-field approach using active beam scanning. (d) A three-field approach in which all fields use active beam scanning, but each field delivers a near-uniform dose to the target volume. (e) One of three fields used in the intensity modulated proton plan shown in panel f. This field alone delivers a highly nonuniform dose across the target volume. (f) A three-field, optimized intensity modulated proton treatment in which the individual fields deliver a highly nonuniform dose across the target volume. The result is a near-uniform, highly conformal dose to the target. Note, also, the high degree of sparing of the bone in the center of the high-dose region.
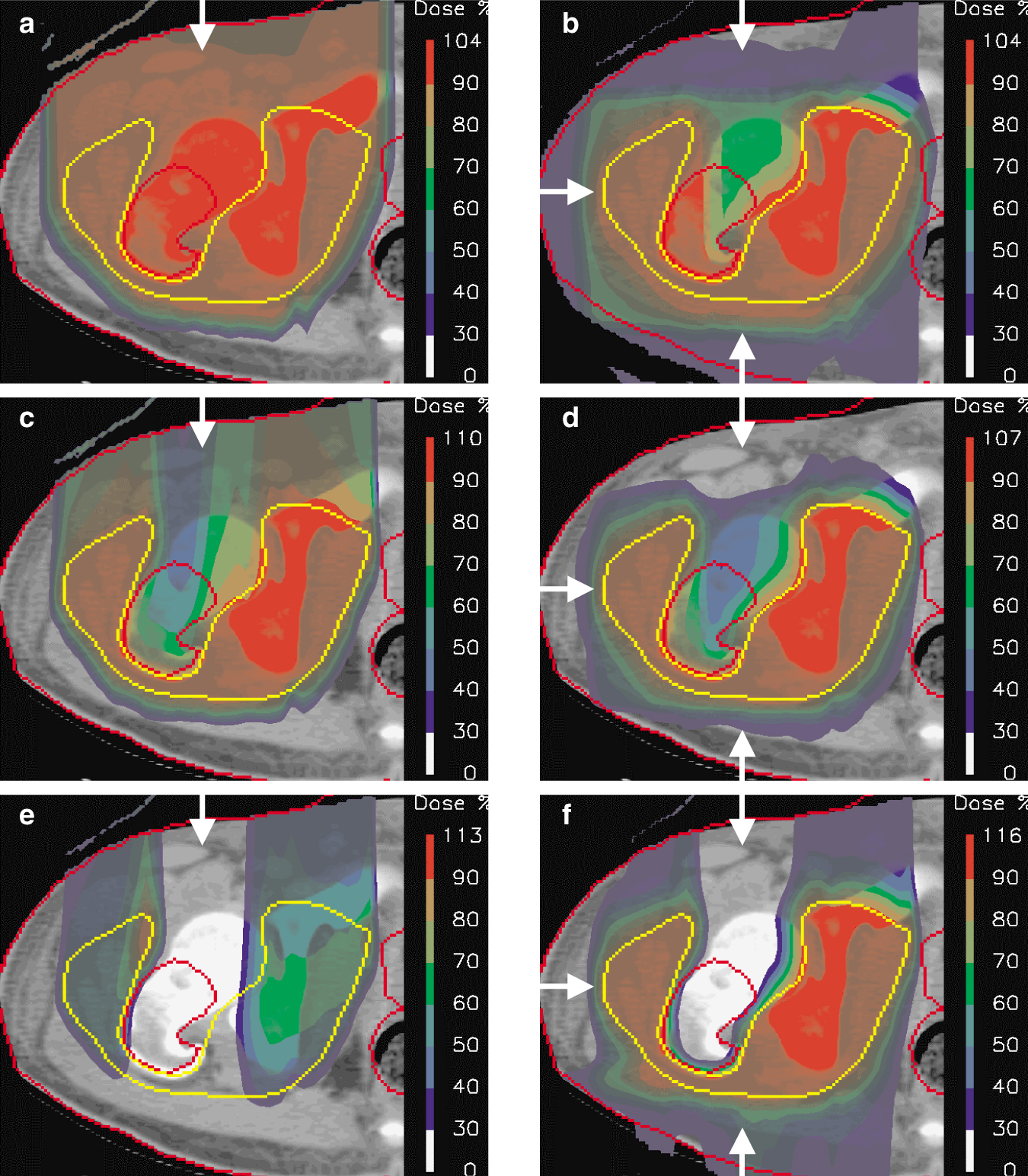
In practice, multiple, angularly separated fields are used to enhance the dose conformation to the target volume (figure
Passive scattering is a simple, effective, and mature method of delivering proton therapy. The history of radiotherapy, however, is one of progression. Developments continue to be made in both the planning and delivery of proton therapy treatments. Although research is proceeding in many directions, one path can be traced through the lineage of proton therapy delivery: a progressive increase in the number of delivery parameters that are modulated.
Passive scattering uses a single order of modulation. That is, its delivery is based on the successive modulation of range-shifted Bragg peaks along each delivered beamlet. This one-dimensional modulation is why the passive scattered field shown in figure 4(a) cannot provide conformation to the proximal side of target: It does not have enough degrees of freedom.
A natural progression from passive scattering is to increase the level of modulation inside a single field. One tactic is to modulate not only the weights of Bragg peaks as a function of depth, but also the relative fluence of individual beamlets across the plane of the delivered field. Such a third-order modulation is the idea behind active beam scanning, in which each field is made up of many (typically thousands) of pencil beams whose energies and lateral positions are chosen so as to locate their Bragg peaks within the target wherever they are needed and whose weights can be individually adjusted. In general, any physically possible dose distribution can be realized by using gradient-based optimization algorithms to determine the appropriate weights. For instance, one may elect to have each field deliver a uniform dose to the target volume while ensuring, in contrast to passive scattering, that the high-dose area of the combined fields conforms also to the proximal surface of the target volume. Figure
Spot-scanning can be used to deliver any desired (and physically possible) dose distribution. In particular, it can deliver intensity-modulated proton therapy (IMPT), just as in intensity modulated x-ray therapy. In IMPT, a uniform dose within the target can be constructed from a number of individually nonuniform proton fields. Strictly, IMPT does not provide an additional degree of freedom, but it does allow the full exploitation of all the degrees of freedom provided by the many thousands of individually weighted and three-dimensionally distributed Bragg peaks. Figure
Passive Scattering and Active Scanning
The upper panel illustrates some of the operating principles of the passive scattering technique for creating spread-out Bragg peaks (SOBPs). In passive scattering, spreading in range is accomplished either through a rotating absorber of appropriately tailored profile or by a ridge filter, whose multiple ridges are machined to interpose a range of appropriate thicknesses of absorber in the beam. Lateral spreading is accomplished by a system of scatterers with various degrees of complexity (the figure shows one of the simplest systems: two scatterers, the second of which incorporates an occluding plug). A more sophisticated approach to lateral spreading is to use a double-contoured second scatterer of two different atomic-weight materials. The goal is to achieve a high efficiency of beam utilization and uniformity while keeping the proton range constant, well defined, and independent of radial position. Specially shaped collimators and compensators further improve the dose distribution by adjusting the proton range to coincide with the distal edge of the target volume. Compensators for each patient are produced individually using computer controlled milling machines.
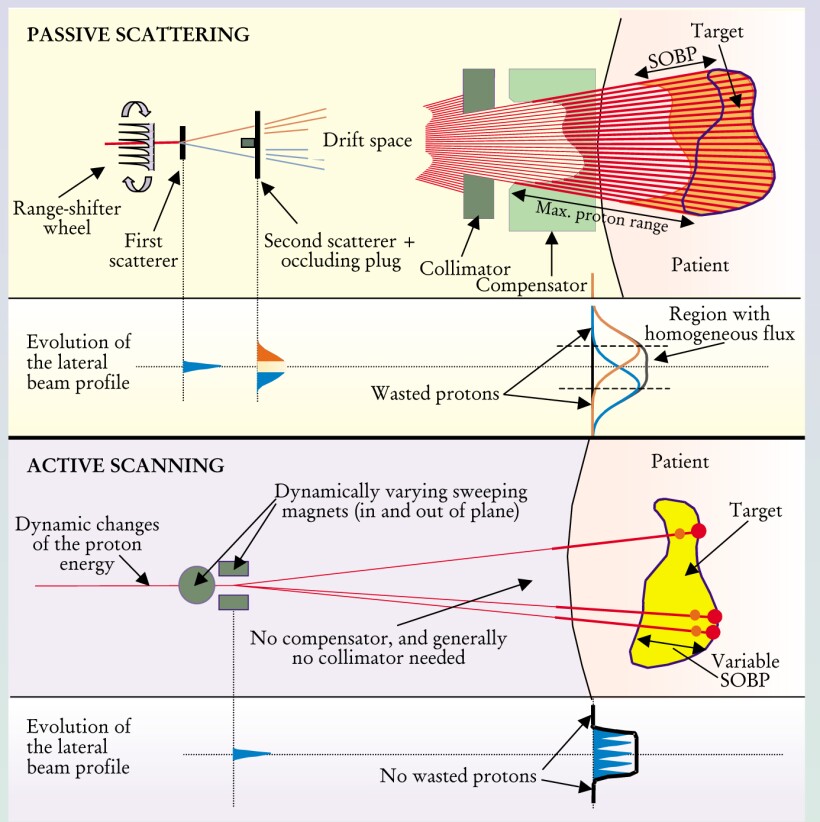
The lower panel illustrates the basic principles of an active scanning system. The proton pencil beam that emerges from the accelerator beam is applied directly to the patient. Lateral spreading is achieved by deflecting the beam with magnets. Adjusting the depth of the Bragg peak is done either by changing the energy of the beam or by dynamically adding material in front of the patient with a range shifter. The scan may be performed in discrete steps (spot scanning) or continuously (raster scanning). By changing the scanning speed or beam intensity, the dose delivered at each point in the target is controlled. The most important challenge for the future is to increase the speed of scanning so that multiple target repaintings can be applied in the same session, thereby making the scanning method more robust against organ motion artifacts.
Clinical experience
The goal of localized therapies such as protein beam therapy is to so damage the malignant cells that the tumor loses its capacity to grow. The likelihood of accomplishing this goal is known as the tumor control probability (TCP).
Since its clinical debut in the late 1950s, proton therapy has been used to treat more than 30 000 patients worldwide. 6 The most widely treated malignancy has been uveal melanoma, a cancer of the retina. Clinical results for uveal melanomas are excellent. For the more than 5000 patients treated for that condition at Massachusetts General Hospital (MGH) in Boston and at PSI, TCPs of up to 98% have been achieved—and this with a 90% chance that the patient retains the treated eye. (The only alternative therapy with a similar cure rate is surgical removal.) Furthermore, in a randomized clinical trial at Lawrence Berkeley National Laboratory, the use of helium ions (whose effect is very similar to that of protons) to treat uveal melanoma yielded a TCP of 100% compared with 87% when using iodine-125 plaques.
MGH has obtained similar impressive results for skull base sarcomas. Ten-year TCPs of 94% and 54% have been achieved for the two major types of skull base sarcomas, chondrosarcomas, and chordomas, respectively. In comparable studies with conventional treatments, TCPs are significantly lower.
At MGH, a randomized clinical trial for prostate carcinoma has been performed, in which treatments with and without a proton boost were compared. A study consisting of more than 200 patients showed a significant improvement in seven-year survival for poorly differentiated tumors in the proton-boosted group (85% compared with 37% for x rays alone). But no significant difference was found over all patients in the study. At Loma Linda University Hospital in California, protons were used to treat a group of more than 600 prostate carcinoma patients. That study has seen tumor control rates at five years of between 100% and 53% (depending on the histological activity).
At the same institute, disease-free survival of up to 83% (for stage 1 patients) has been achieved in the treatment of lung cancer. A facility in Chiba, Japan, has achieved similarly excellent results in the treatment of lung tumors with carbon ions at remarkably high doses. Another Japanese team, from Tsukuba, has reported encouraging results for the proton irradiation of hepatocellular carcinomas at high doses. The more than 120 patients in the Tsukuba study experienced TCPs of more than 90%.
Many smaller studies have investigated the therapeutic potential of protons. Examples include paranasal sinus carcinomas (89% TCP at 3 years), benign meningiomas (93% survival at 10 years), atypical and malignant meningiomas (80% TCP at 5 years), and sarcomas of the spine (between 100% and 54% TCP depending on the histology). When delivering dose to the spine from the posterior aspect, the finite range of protons is a clear and obvious advantage over conventional photon therapy for sparing the thoracic and abdominal organs.
As proton therapy becomes more widely available in hospitals, the range of conditions treated will certainly increase. One underexplored area is pediatric cancer. Because they are still growing and evolving, a child’s organs are more susceptible to radiation damage than an adult’s. Thanks to its ability to spare healthy tissue, proton therapy will almost certainly make a large impact in the treatment of childhood tumors—particularly by reducing untoward side effects. Treating very young children requires anesthetics, so the move toward hospital-based proton therapy centers is a prerequisite for these patients.
Like children, patients who undergo combined treatments (chemotherapy and radiation therapy, for example) are also particularly sensitive to the burden that treatment places on healthy tissue. Such patients are potentially more susceptible to adverse reactions to either of the treatment modalities. The reduced doses delivered by protons to healthy tissue could improve patients’ tolerance to combined treatments. For the optimal management of such treatments, hospital-based facilities are necessary.
Conclusions
Protons have come of age as a clinical tool. They have moved from the laboratory to the clinic, and from an obscure activity to a real possibility for healthcare professionals wishing to provide the most advanced care for their patients. The routine implementation of IMPT will raise proton beam therapy to the physical limit of its possibilities. Nevertheless, as we have tried to point out, important challenges remain that should make proton therapy a fruitful field for physicists for some time to come.
The economics of proton therapy are critical—and still quite unclear. At present, the cost of a proton beam facility is at least a factor of three higher than a conventional radiation facility of comparable capacity. However, operational costs dominate the cost of treatment. Those costs are expected to come down once proton therapy has achieved a higher degree of operational efficiency than it now has. Most people are persuaded that, if it cost no more than x-ray therapy, proton therapy would be preferred. The issue, then, is one of cost-effectiveness. It cannot be resolved at this time because we do not know either proton therapy’s relative cost or its relative effectiveness. However, widespread clinical evaluation is just around the corner. It will be fascinating to see where it leads.
References
1. R. R. Wilson, Radiology 47, 487 (1946).
2. M. R. Raju, Heavy Particle Radiotherapy, Academic Press, New York (1980).
3. Ute Linz, ed., Ion Beams in Tumor Therapy, Chapman & Hall, New York (1995).
4. A. J. Lomax, M. Goitein, in Advances in Hadrontherapy, U. Amaldi, B. Larsson, Y. Lemoigne, eds., Elsevier Science BV, New York (1997), p. 233.
5. U. Amaldi, B. Larsson, eds., Hadrontherapy in Oncology, Elsevier Science BV, New York (1994). (The book is a good overview of the field; beam delivery is treated very generally from page 435 on.)
6. I. J. Spiro, A. R. Smith, A. J. Lomax, J. S. Loeffler, in Cancer: Principles and Practice of Oncology, 6th edition, V. T. DeVita, S. Hellmann, S. A. Rosenberg, eds., Lippincott, Williams & Wilkins, Philadelphia (2001), p. 3229.
More about the authors
Michael Goitein (michael@goitein.ch
Michael Goitein, (michael@goitein.ch) Harvard Medical School in Boston, Massachusetts, US.
Antony J. Lomax, Paul Scherrer Institute in Villigen, Switzerland.
Eros S. Pedroni, Paul Scherrer Institute in Villigen, Switzerland.




