The wonderland of angstrofluidics
DOI: 10.1063/pt.frik.vxpk
Fluids are everywhere. They are the water we drink, the air we breathe, and the blood that flows through our bodies. In nature, water striders seem to defy physics, but they simply combine surface tension of the water with the hydrophobicity of their legs to walk on water. Fluids have unique properties, including surface tension, viscosity, and capillary action, that engineers have taken advantage of to create practical applications, such as reverse osmosis, inkjet printing, and DNA sequencing.
The bulk properties of fluids are usually explained by the Navier–Stokes equations, which connect various fluid parameters, including density, pressure, and velocity. The equations describe exceptionally well the behavior of fluids at the macroscale—for example, the airflow around an airplane or the water in a kitchen tap. But the equations break down at the molecular scale.
The Navier–Stokes equations assume that the fluid is in the hydrodynamic limit, in which the continuum framework holds and the measurement scale is large enough that the tiny details of molecular movement average out. That means that the fluid’s behavior can be described using a few measurable properties. The lowest scale at which the continuum framework remains valid is theoretically around 1 nm, or 10 Å. The characteristic signs of the breakdown of the continuum framework include liquid structuring and single-file transport, which lead to seemingly frictionless enhanced flows observed in angstrom-scale channels.
It would be reasonable to assume that fluids move sluggishly through angstrom-scale channels. Yet astonishingly fast flow rates of 1 billion molecules per second have been reported. Channels, pores, and other types of openings that are less than 1 nm fall within the angstrofluidics domain. Openings between 1 nm and 100 nm fall within the nanofluidics domain. At those minuscule scales, the effects of individual molecular interactions and high surface-to-volume ratios become more important.
Those effects lead to intriguing properties of fluids at molecular scales. The properties are more than just scientific curiosity; they can be adapted for practical applications. For instance, nano- and angstrom-scale channels can be used to mimic the kidney’s filtering capacity in an energy- and cost-efficient manner. 1 Given that more than 2 billion people worldwide still don’t have access to safe drinking water, the potential use of nanofluidics in water desalination holds immense promise to help solve real-world problems. Research into nanofluidics-assisted computing and memory devices expands the potential applications. 2 , 3
The fields of nano- and angstrofluidics build on the nanofabrication expertise developed for semiconductors. Recent breakthroughs in fabrication capabilities and 2D materials have pushed angstrofluidics even further, so there has never been a better moment for anyone interested in the field to get started.
History and background
The basic building blocks of fluids are molecules that have a weaker attraction between them than the molecules observed in solids. That allows fluids to circulate freely and diffuse throughout a container, like water filling a glass or perfume spreading throughout a room. The ability of molecules to migrate from one place to another is quantified by the diffusion coefficient, whose value depends on the specific fluid, concentration, and temperature. A related term—mobility—characterizes how easily molecules can move under different stimuli.
For illustration, consider the case of a salt solution kept in two chambers connected by a small hole. A battery connected across the chambers will create an electric field in the liquid. In such a case, the electric field will drive positively charged ions to migrate through the hole in the direction of the field, and the negatively charged ions will move in the opposite direction. The ions’ speed is determined by their mobility. The Nernst–Einstein relation explains the connection between the diffusion coefficient and mobility.

Timeline of nano- and angstrofluidics. Many science disciplines overlap in the study of nano- and angstrofluidics. Tiny channels for moving ions were discovered in cell membranes in the 1970s, and later, studies of protein channels revealed their sequencing capabilities. Researchers have taken inspiration from nature and have used tools developed for other fields to create artificial channels, progressing quickly from carbon nanotubes to 2D angstrom-scale channels. (Adapted from refs.
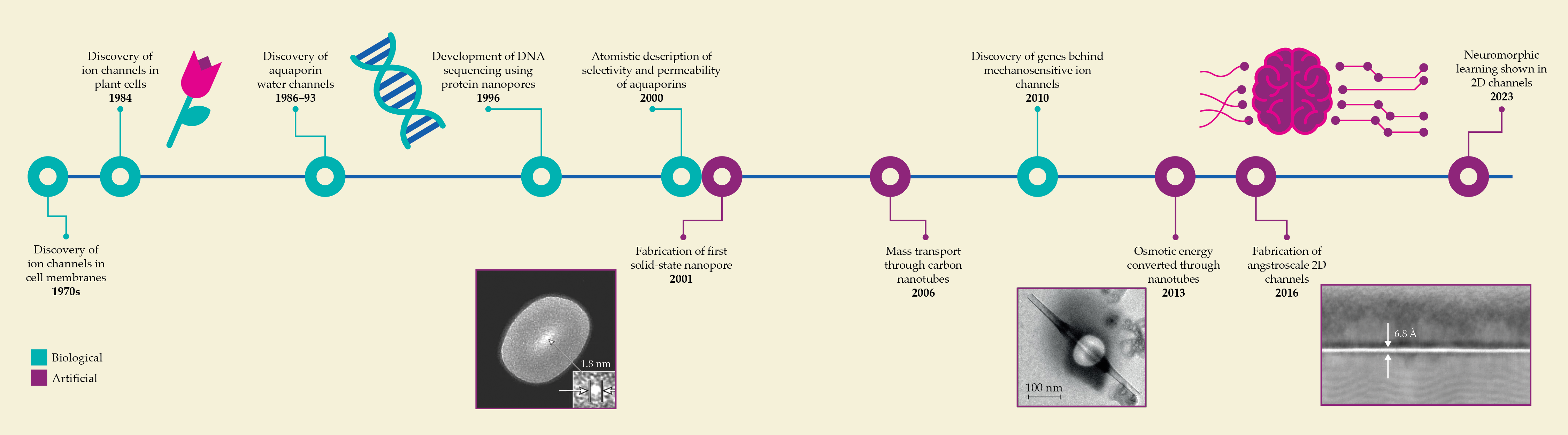
The dielectric constant determines how the electric field lines will be distributed. The magnitude of the dielectric constant is proportional to the ability of fluid to store electrical energy. Even though the bulk dielectric constant of water is approximately 80, confined water in angstrochannels has a suppressed dielectric constant of approximately 2, which arises from inhibited rotational motion of water molecules. 4
Even though nanofluidics was initially explored as a scaled-down version of macrofluidics, its exceptional capabilities and properties led to its emergence as a separate field. Advances in theory, characterization, and fabrication have contributed to the growth of nanofluidics in the past 20 years. Engaging with the mysterious universe that exists at the smallest scales requires specially designed devices and tools. Nanofabrication developments adapted from semiconductor engineering enabled the creation of nanopores, 5 , 6 nanotubes, 7 and nanoslits. 8 , 9 Those three primary classes of devices, with their different channel dimensionalities, have enabled researchers to conduct ionic-transport experiments from which they have learned the properties of nanofluidics and developed related applications.
Understanding the nano- and angstrom-scale fluidic properties requires coupling measurement techniques with channel devices. The primary characterization tools used in the nanofluidics domain include electrochemical and electrical measurements, spectroscopy, gravimetry, and various microscopy techniques. Although those methods have been essential to the progress of nanofluidics research, they each have drawbacks. As a mechanical method, atomic force microscopy is limited by the influence of the tip perturbation on the liquid, and related capillary forces affect the measurement. Optical microscopy is hindered by diffraction limits, but transmission electron microscopy cannot always be used because a vacuum is needed. New types of characterization techniques are being developed to overcome those limitations.
Innovation through confinement
A ball rolling smoothly across a vast football field will follow a path that is relatively uninhibited. But what if the ball were navigating a maze? The walls would guide, block, and even divert it. That is analogous to the world of nano- and angstrofluidics, in which fluids interact intimately with the structures confining them.
At such small scales, the structures aren’t passively containing the fluid; they actively influence its behavior. A maze’s walls might have slopes or turns that change how the ball moves. Similarly, the surfaces of a nanochannel might be rough, charged, or embedded with specific binding sites. Those features can drastically affect the fluid’s flow and interactions. For instance, a nanochannel’s electrostatic charges or specialized binding sites might interact selectively with only certain molecules. And the channel’s tight dimensions can act as selective gates that determine which molecules pass through and in what orientations. That isn’t just a challenge—it’s an opportunity. We can harness those interactions to design devices with specific functionalities, such as selective filtration or ion-charge discrimination.
Charges on the walls of a nanofluidic channel attract ions of opposite polarity, and the accumulated ions form an electrical double layer. It is made up of the immobile Stern layer, with tightly bound ions, and the diffusion layer, with loosely bound ions. When a voltage difference is applied across a channel, the flow in the diffuse layer causes increased ion-specific mobilities in the nanochannel. The interaction between the electric double layer and the ions that are flowing through the channel results in ionic selectivity and filtration.
In angstrofluidic channels, however, wall–fluid interactions, ion-pair formation, and other phenomena lead to unique properties such as ionic streaming currents, size selectivity, and ionic memory. The new opportunities make angstrofluidics research much more breathtaking. Recent studies have examined quantum interactions between neutral molecules—for example, water confined at the nano and angstrom scales—and electronic excitations in the solid walls of channels. 10 Water flowing in a channel can induce an electronic current in the solid wall because of hydroelectronic drag, and that can be harvested as hydroelectricity from small-scale water flows. For salt solutions flowing in nano- and angstrofluidic channels, ion–ion and ion–surface interactions can lead to osmotic energy generation and neuromorphic computing applications. 2 , 3 Today all computers function fundamentally by controlling the electron flow through solids. Imagine a time in the future when scientists create saltwater-based computers inspired by the working of our brains!
Angstrom-scale behavior, though, isn’t always different from macroscale behavior. Capillary condensation occurs across the vastly different scales. The phenomenon can be utilized in nanoporous materials to collect clean water from the atmosphere, a useful trick in deserts and other extreme environments. Another example of common behavior at the nano and angstrom scales is gas diffusion in the Knudsen regime. The Knudsen equation describes how gases move through tiny holes that are smaller than the average distance a gas molecule travels before colliding with another molecule. This equation remains accurate even for apertures that are just a few atoms wide. 9
Real-world applications
Although angstrofluidics is a recent field of study, many applications aren’t hard to imagine, especially those that build off existing nanofluidic uses. Water-filtration membranes are promising because angstrom-scale channels have the unique ability to filter based on ionic size and charge while still maintaining high permeability. 11 Already, larger nanofluidic channels are used in desalination devices. Researchers are currently working on a practical desalination apparatus with angstrom-sized channels, but the challenge is to prevent clogging and fouling of the extremely tiny channels. Scientists also are developing efficient carbon capture and filtering technologies using nanoporous membranes.

Nanofluidic devices and their macroscale analogues. (a) A nanopore in a silicon nitride membrane
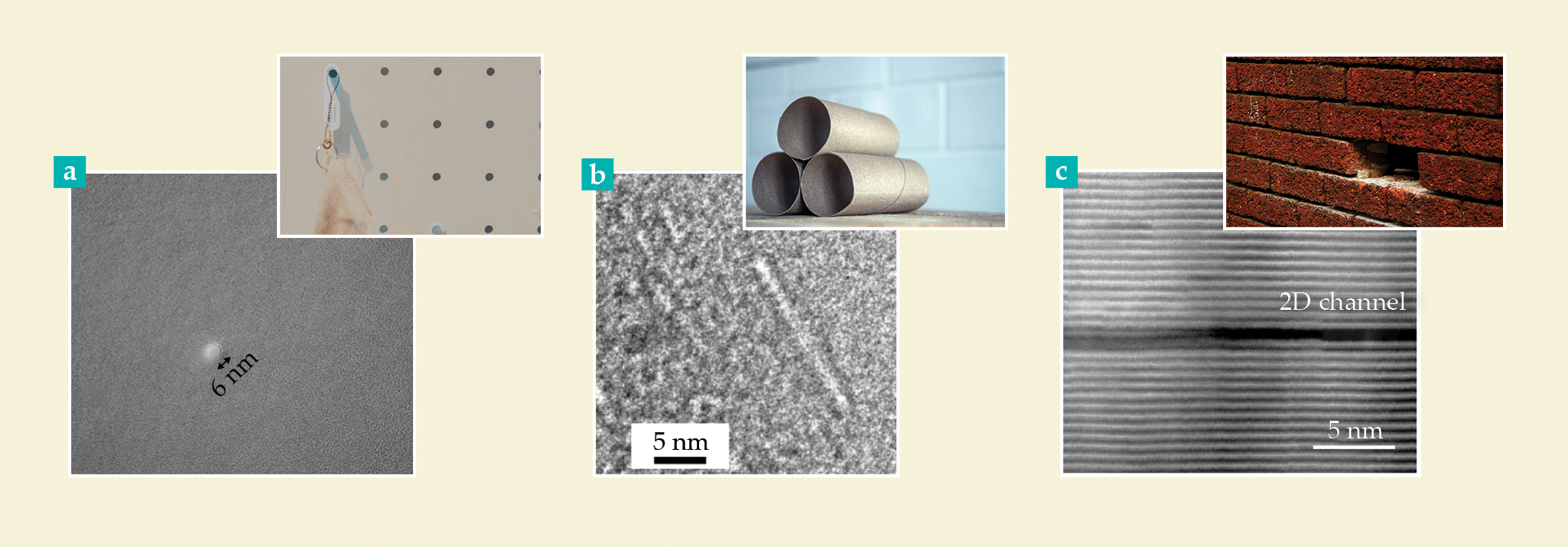
Both nano- and angstrofluidic channels are prominent areas of research for biomedical diagnostics. Currently, nanopores are used to sense and sequence DNA and proteins for therapeutic applications. 12 Parallel efforts are underway to develop minuscule organ-on-a-chip and lab-on-a-chip devices encompassing nanochannel pathways. They have the potential to revolutionize pharmacological research by mimicking the human physiological environment on the microscale, thus enabling more accurate drug testing without the need for animal models. In drug delivery, nano- and angstrofluidic technologies are being harnessed to engineer controlled-release mechanisms and improve the delivery efficiency of therapeutic agents, especially in targeting hard-to-reach cancerous cells. Not only are nano- and angstrofluidic technologies more compact and efficient, but they also have the potential to be more cost-effective, which makes them an especially valuable tool in democratizing health care.
Remote areas can also benefit from energy storage and generation via nanochannels. Ion-selective nanochannels can be placed between solutions of different salt gradients to allow the continuous movement of specific ions. That leads to constant generation of an ionic current. Although not in use commercially at a large scale, such so-called blue-energy generation has been demonstrated at the intersections of seas and rivers. Membranes with angstrofluidic channels will have improved selectivity and thus will lead to efficient osmotic energy harvesting. Angstrofluidics may also offer a way to improve the material and design of batteries, a research goal that covers many disciplines.
Current bottlenecks
Before we can fully use nanofluidic and angstrofluidic channels in large-scale industrial deployment, certain obstacles need to be addressed. Some of the primary bottlenecks are in imaging techniques, fabrication, and the general knowledge gap.
To reveal the mysteries at the molecular scale, new measurement techniques capable of capturing angstrom-scale dynamics are necessary. Several characterization tools are currently being explored, including simple patch-clamp amplifiers to measure ionic currents and high-end microscopes. Yet, as previously discussed, those techniques have drawbacks and limitations. New devices, with better localization than is currently possible, are needed to detect changes happening within subnanosecond intervals.

Blue-energy generation, or osmotic power, is one of many potential real-world applications for nano- and angstrom-scale fluidics. Ion-selective nanochannels can be placed at the intersections of seas and rivers to generate a constant ionic current—a clean energy source. As with the use of nanoporous materials to collect clean water from the atmosphere in extreme environments, the technology has been demonstrated and is waiting on fabrication advancements to be put to use on a wider scale.
STEPHENGG/FLICKR

Tools to explore the interfaces between two phases of matter—liquid and the interacting solid surface—are also necessary. New and old techniques will need to be combined and correlated to allow multifaceted simultaneous measurements and imaging. And because all the phenomena explored here are at the molecular scale, noninvasive measurement techniques need to be developed.
Even though nano- and angstrom-scale channels have the potential to be used in several real-life applications, there are currently no methods to scale up the fabrication of nanochannels for wide use without affecting device functionalities. For example, an industry-scale deployment of nanofluidic filters with molecular selectivity and high permeation to extract uranium and lithium from seawater would be highly beneficial. It requires thousands of device arrays, however, and with the current manufacturing capability, it is a nearly impossible task. Scalable fabrication techniques for nanochannels with anticlogging and antifouling properties are needed. Current developments, such as rapid fabrication techniques that use membrane protein nanosheets, are a step in that direction.
In biology, aquaporin—a type of protein channel—can allow one water molecule through at a time and still achieve transportation of a billion molecules in a second. The human kidney has excellent filtration. Ionic pumps in neurons are extraordinarily fast and sensitive. Creating angstrochannels with comparable capabilities is still a distant dream.
Biological channels also are extraordinarily multifunctional. In addition to offering ion selectivity, they respond to different stimuli, control flow rate, and are highly adaptable. In contrast, artificial nanochannels have to date focused only on specific tasks. Chemists, electrical engineers, and materials scientists working together can provide the interdisciplinary expertise needed to develop nanochannels that mimic the capability of biological pores and go beyond single-purpose devices.
The importance of working with what you have
Because nano- and angstrofluidic devices are at the tiniest scales and involve several key elements—for example, force fields and complex ion-wall interactions—many aspects are yet to be fully understood. For example, the liquid molecules flowing closest to the channel surface are classically expected to have zero velocity, but that may not always be true in angstrofluidic channels. A supersmooth channel wall instead leads to an increase in the fluid velocity, but researchers do not yet have a complete understanding of the mechanisms behind it.
Studies in carbon nanotubes have observed water freezing above 100 °C. And the effect of channel defects on fluid transport is puzzling. Using existing tools and devices, researchers are working on the fringes of what’s possible to decipher the secrets of nano- and angstrofluidics. It is not always the most efficient way to fill in knowledge gaps, but persistence is what keeps the field moving forward.
Even after 20 years of nanofluidics research, the field continues to surprise as groundbreaking discoveries reveal extraordinary properties, especially with angstrom-scale channels. As William Shakespeare wrote in Hamlet, “There are more things in heaven and earth … than are dreamt of in your philosophy.” Likewise, there are undoubtedly more phenomena to be understood in the angstrofluidics domain that researchers have yet to imagine. The future of the field mostly resides in technological developments and fabrication advancements. The applications span disciplines, and collaboration across the sciences will yield many benefits. Progress will require many more curious researchers ready to delve into the field and join the exciting search to unravel the mysteries of nano- and angstrofluidics.
This work was supported by the Royal Society University Research Fellowship, a European Union H2020 ERC Starting Grant (852674—AngstroCAP), the Philip Leverhulme Prize (PLP-2021-262), an EPSRC New Horizons grant (EP/V048112/1), and a Commonwealth Split-Site PhD Scholarship. We are grateful to everyone who contributed to the research on angstrom-scale fluidics.
References
1. S. Marbach, L. Bocquet, Phys. Rev. X 6, 031008 (2016). https://doi.org/10.1103/PhysRevX.6.031008
2. P. Robin et al., Science 379, 161 (2023). https://doi.org/10.1126/science.adc9931
3. T. Emmerich et al., Nat. Electron. 7, 271 (2024). https://doi.org/10.1038/s41928-024-01137-9
4. L. Fumagalli et al., Science 360, 1339 (2018). https://doi.org/10.1126/science.aat4191
5. J. Li et al., Nature 412, 166 (2001). https://doi.org/10.1038/35084037
6. A. Dominic et al., J. Vac. Sci. Technol. B 41, 043204 (2023). https://doi.org/10.1116/6.0002682
7. A. Siria et al., Nature 494, 455 (2013). https://doi.org/10.1038/nature11876
8. B. Radha et al., Nature 538, 222 (2016). https://doi.org/10.1038/nature19363
9. A. Keerthi et al., Nature 558, 420 (2018). https://doi.org/10.1038/s41586-018-0203-2
10. N. Kavokine, M.-L. Bocquet, L. Bocquet, Nature 602, 84 (2022). https://doi.org/10.1038/s41586-021-04284-7
11. A. Esfandiar et al., Science 358, 511 (2017). https://doi.org/10.1126/science.aan5275
12. H. Brinkerhoff et al., Science 374, 1509 (2021). https://doi.org/10.1126/science.abl4381
13. R. H. Tunuguntla et al., Nat. Protoc. 11, 2029 (2016). https://doi.org/10.1038/nprot.2016.119
More about the authors
Radha Boya is a professor and Royal Society University Research Fellow in the department of physics and astronomy and Ashok Keerthi is a Presidential Fellow in the department of chemistry at the University of Manchester in the UK. Muhammad Sajeer Parambath is a joint PhD scholar at the University of Manchester and the Indian Institute of Science Bengaluru.







