The physics of lipidic mesophase delivery systems
DOI: 10.1063/PT.3.4522
An ideal drug delivery system should be biodegradable and biocompatible and should incorporate the active pharmaceutical ingredient without losing or altering its activity. To optimize the drug’s therapeutic time window, the system should provide an efficient and controlled release mechanism for a drug at a specific location in vivo. Lipidic mesophases, a special class of materials belonging to the lyotropic-liquid-crystal family, meet those criteria. They thus share characteristics of both crystals and liquid materials: Like crystals, their molecules have short-range positional order, and like liquids, they lack long-range positional order. Lipidic mesophases exhibit distinct structures based on their composition, and they constitute an attractive alternative drug delivery system, capable of incorporating molecules of different polarity and size and protecting them from chemical and physical degradation.
As figure
Figure 1.

The lipid mesophase structures shown here offer a means to protect and transport drugs through the human body. The inverse bicontinuous cubic phases can display three different symmetries: the double gyroid (Ia3d) has threefold connectivity of the aqueous channels; the double diamond (Pn3m) displays fourfold connectivity; and the primitive (Im3m), sixfold connectivity. The inverse micellar cubic phase comprises spheroidal micelles arranged in Fd3m or Fm3m symmetry. (Adapted from ref.
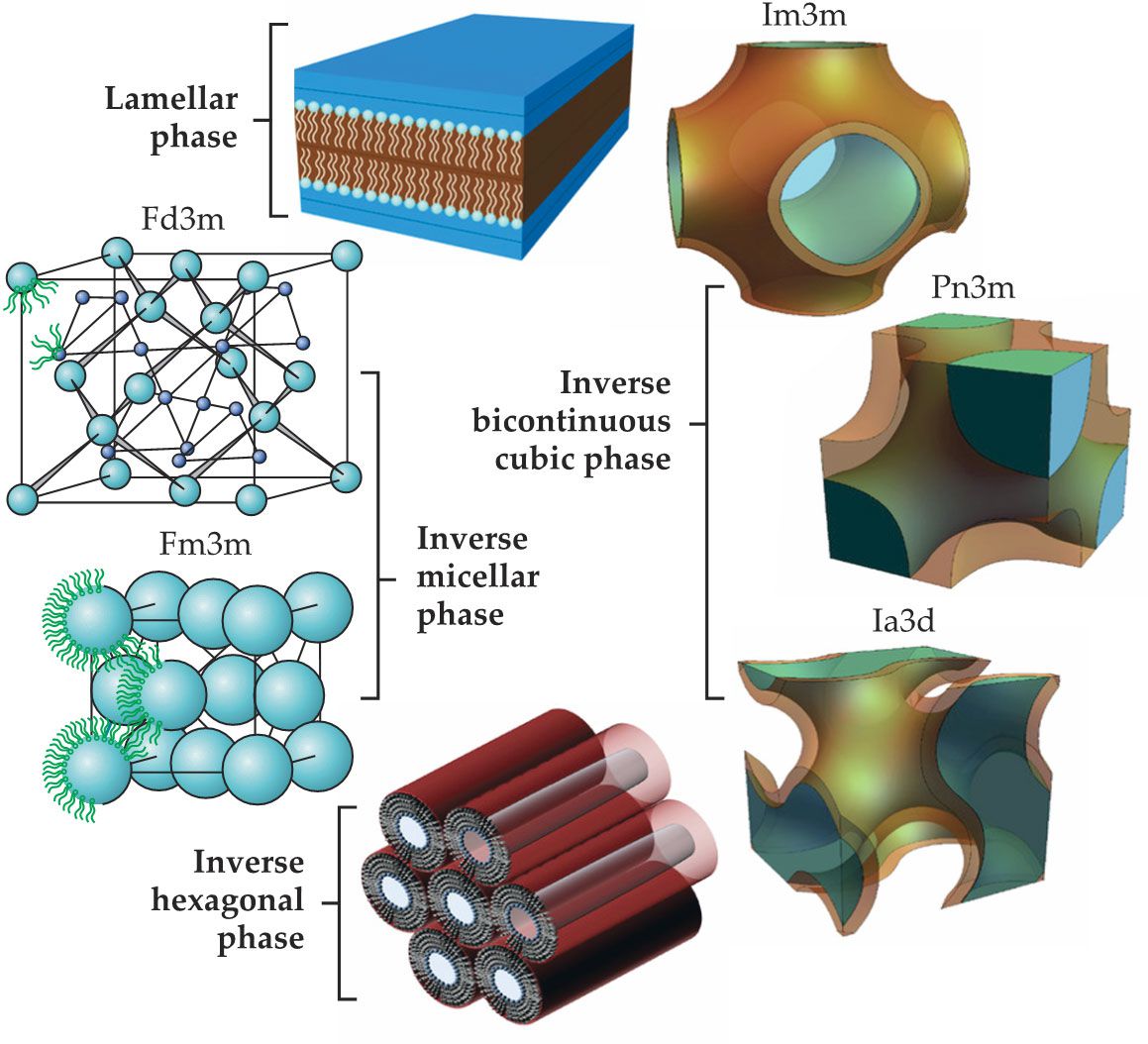
The bicontinuous cubic phases are composed of two sets of water channels separated by curved bilayers in the 3D space such that every point on the bilayer midplane surface is a saddle point with a zero-mean curvature. 2 Those bilayers encompass a system of aqueous channels and form structured yet flexible networks that are nonbirefringent and optically transparent. Specifically, lipid molecules in bicontinuous cubic phases form a highly curved continuous bilayer that separates the two interpenetrating but nonintersecting aqueous channel networks. The structures can display a double gyroid (Ia3d, with threefold connectivity of aqueous channels), double diamond (Pn3m, with fourfold connectivity), and primitive (Im3m, with sixfold connectivity) symmetry. 3 Finally, the inverse micellar phase comprises spheroidal micelles arranged in Fd3m or Fm3m symmetry, or in a disordered state L2. Most lipids do not show those phases in pure water and need oil-based components, such as oleic acid or limonene, to form the micellar cubic phase.
The critical packing parameter CPP—defined as the ratio between the hydrophobic chain volume and the hydrophobic chain length in the molten state times the cross-sectional molecular area—can predict qualitatively the structural phase given by each lipid in the presence of water. However, because of the complexity of bicontinuous cubic phase structures, CPP theory cannot fully describe the system.
4
Lipids, such as phytantriol (PHT), monoolein (MO), and monolinolein (MLO), and phospholipids are the most well-known macromolecules capable of forming lyotropic liquid crystals in water via self-assembly. MO and MLO, which are generally recognized as safe for human and animal use by the US Food and Drug Administration, are the most widely used materials for encapsulating a broad range of drugs with various sizes and polarities. Their phase diagrams, shown in figure
Figure 2.

The phase diagrams of the lipids (a) monoolein, (b) phytantriol, and (c) monolinolein show the different structures each can form at thermodynamic equilibrium for different water compositions. Two symmetries are possible for the lamellar phase: the crystalline lipid tail form LC, also known as Lβ, and the amorphous lipid tail form Lα; three for the inverse bicontinous cubic phase (Ia3d, Pn3m, Im3m); and three for the micellar cubic phase—Fd3m and Fm3m if the micelles are organized in a lattice and L2 if in a disordered state. (Adapted from ref.
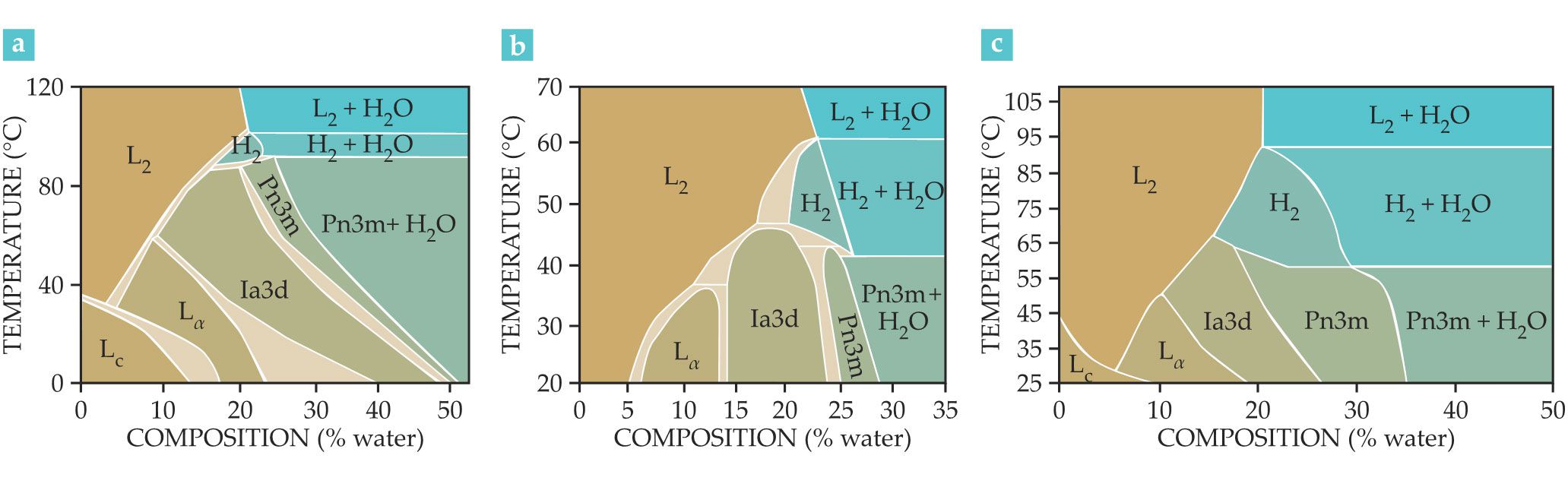
Once the maximum hydration level has been reached, the Pn3m phase cannot swell anymore. The excess water given to the system remains confined outside the cubic structure, and the Pn3m bicontinuous cubic phase coexists with excess water at thermodynamic equilibrium. The Im3m bicontinuous cubic, hexagonal, and the micellar Fm3m phases show a similar behavior: Excess water coexists thermodynamically with lipidic mesophases.That behavior is a crucial and distinctive feature of lipid mesophases and makes them valuable as drug delivery systems. The thermodynamic equilibrium allows researchers to design nanostructures that remain stable for the entire drug-delivery process, which makes lipid mesophases more advantageous compared to other nanostructured fluids, such as thermodynamically unstable nanoemulsions. 5
When mesophases coexisting with excess water have reached their maximum hydration level, a dispersion of bulk phases can be formed by adding energy to the system. For sonication, the most commonly used approach to form dispersions, a lipid film is hydrated in the desired buffer containing a stabilizing polymer, and sound waves agitate the film until the sample becomes a homogenous dispersion. 6 The water-dispersed forms of the parent cubic- and hexagonal-phase bulk counterparts are cubosomes and hexosomes. They possess the same nanostructure as the parent bulk phase but exhibit a much lower viscosity, improved fluidity, and larger surface area. Such dispersions are commonly prepared by mixing lipids with a stabilizing polymer that acts as a surfactant and improves the nanostructure’s shelf life. With those dispersions, researchers can deliver poorly water-soluble drugs, selectively target malignant cells, and use molecular detection to identify and deliver radioactive drugs for radiation therapies. 7
Phase characterization techniques
Researchers routinely use small‐angle x-ray scattering (SAXS) to determine the lipid phase, and thus construct the phase diagram, for different lipid–water systems. That method relies on constructive interferences in the reciprocal space from many ordered scattering planes that belong to the mesophase. An x-ray beam is directed at the lipid sample, and the resulting scattering pattern gives a characteristic set of rings, or maxima, that correspond to Bragg reflections. Their positions in the reciprocal space depend on the Miller indices of the mesophase scattering planes, and the sequence of Bragg reflections consequently identifies the symmetry of the mesophase studied. SAXS allows for the lattice parameter—the size of the repeat unit cell—to be determined. When the parameter is translated, researchers can reconstruct the entire mesophase in 3D. The lattice parameter and the phase composition enable scientists to determine structural features of the mesophase, such as the width of a lipid bilayer and the diameter of water channels, micelles, and slabs.
Besides SAXS, cryogenic transmission electron microscopy (cryo‐TEM) also characterizes lipidic mesophases. Rather than probing reciprocal space, it uses direct measurements of real-space structures. Using cryo-TEM, scientists have obtained high‐resolution images of bicontinuous cubic mesophases, which enables direct visualization of their internal channels, size, and other important structural information. Since SAXS probes structure in the reciprocal space and cryo-TEM in real space, taking the Fourier transform of the lipid structures in the cryo‐TEM images results in a phase assignment equivalent to that obtained via SAXS. 8
Rheology of lipidic mesophases
One of the first features to consider when designing drug delivery systems based on lipidic mesophases is the rheological behavior of the mesophases, which varies tremendously. Measurements of the storage moduli G′ and the loss moduli G″ as a function of the shear frequency ω reveal important information about the rheological behavior of the mesophases. Different viscoelastic regimes emerge because of the diverse topologies of the mesophases, and each regime’s water–lipid interface has its own specific relaxation time. The viscosity of mesophases increases progressively from the less viscous lamellar phase to the viscoelastic hexagonal and the highly elastic bicontinuous cubic phases. 9 Specifically, the lamellar phase behaves as a plastic fluid that has extensive energy dissipation mechanisms due to the parallel slip of the lamellae. The hexagonal phase behaves as a viscoelastic material with a relaxation time of about 0.5–1 s. In contrast, the bicontinuous cubic phases show complex rheological behavior simulated by a multiple Maxwell model. Those phases have characteristic times of up to several tens of seconds that are associated with the relaxation of the lipid head group–water interface. The variation of the relaxation time as a function of the temperature or composition captures subtle cubic-to-cubic phase transitions and the more trivial cubic and excess-water transition.
Although the viscous cubic phase has an optimal rheology to act as a drug depot, its high viscosity could make the subcutaneous administration of drugs challenging. Water can overcome that challenge by triggering the in situ formation of gelled lipidic structures, starting from a liquid or less viscous material. 10 For example, a technology developed by the Swedish company Camurus dissolves the drug in a lipid solution that transforms to gel upon contact with water. After subcutaneous injection, the precursor lipid solution swells from the water naturally present in the tissue and forms the lipid depot in situ.
Connecting mesophase structure and drug release
Mesophases can slowly release hydrophobic or hydrophilic biomacromolecules such as proteins, antibodies, RNA, and DNA. But some small hydrophilic drugs encapsulated in the water channels of a mesophase burst out of it. So far, the most commonly explored approaches to control a hydrophilic drug’s release from lipidic mesophases include ionic interactions between the drug and the lipid polar group, variation of the water-channel dimensions, and the formation of a lipid prodrug, a compound that activates in the body to produce a working drug.
The release processes of hydrophilic molecules depend on the structure of the mesophase. The release phenomenon—a Fickian diffusion process—follows a first-order kinetic profile regulated by the size of the mesophase’s aqueous channels, the size of the drug, and most importantly, the symmetry of the mesophase. To be released, hydrophilic drug molecules must diffuse through the water domains and cross the lipid domains. The ability of the lipids to keep a drug confined in the water channels controls its release. Drug diffusion depends on the dimensionality d of the water domains, defined as the number of orthogonal directions a molecule may explore without leaving the water channel.
In the micellar cubic mesophase, the drug is confined in the micelles with water domains of about 2 nm in diameter (d = 0). In a hexagonal phase, the molecules move through cylindrical water channels (d = 1), whereas in a lamellar phase they can move along the planar bilayers (d = 2). In bicontinuous cubic phases, the diffusion is a 3D process (d = 3) in which the mobility of embedded hydrophilic molecules is hindered only by the channel size—about 5 nm in diameter—and its tortuosity. In general, the diffusion coefficient of a hydrophilic drug decreases with the dimensionality of the phase. Therefore, the Pn3m phases (d = 3) show the highest diffusion coefficients, whereas the micellar cubic phases (d = 0) show the lowest, as illustrated in figures
Figure 3.

The diffusion coefficient (a) of hydrophilic drugs increases with the dimensionality of the lipidic mesophase. (b) Different phase geometries (solid and dashed lines) affect the rate at which a drug is released from the lipid. The diffusion coefficient is also affected by the structural control efficiency index (SCEI). (c) The blue line illustrates a strongly structure-controlled system, such as one composed of monolinolein (MLO), hexadecane, and glucose or a system of MLO, tocopherol, and glucose. The SCEI is about 1, which means on average the diffusion coefficient increases by an order of magnitude per additional dimension. On the other hand, for weakly structure-controlled systems, such as phosphocholine-limonene-caffeine, illustrated by the red line, the SCEI is close to zero. In that case, the hydrophobic lipid domain shows high permeability to the hydrophilic drug. (Figure by S. Aleandri and R. Mezzenga.)
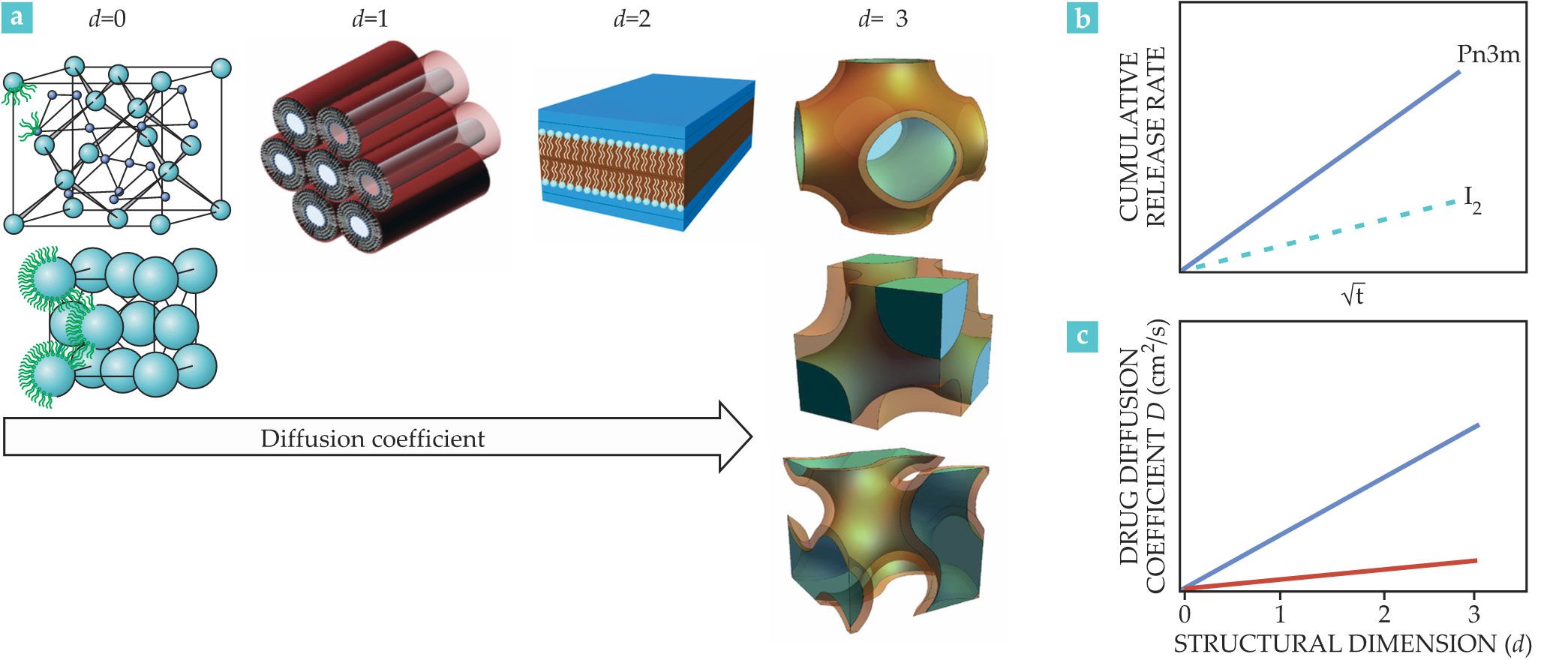
Researchers in 2015 introduced the structural control efficiency index (SCEI).
11
It provides an estimate of the kinetics of drug release for various phases and hydrophobic domains, which have different symmetries and permeability to drugs. The SCEI = log (Dmax / Dmin) / DS, where Dmax and Dmin are the highest and lowest diffusion coefficients observed for a given system, and DS is the structural dimension span explored; DS = 3 for MLO-based systems, whereas DS = 2 for phosphatidylcholine-based systems. Figure
For a given drug–lipid pair with the same dimensionality, topology controls the diffusion process. Diffusing molecules, for example, interact with the geometrical attributes of the three main symmetries of inverse bicontinuous cubic phases—Pn3m, Ia3d, and Im3m—which make a pivotal contribution in determining the diffusion mechanism. For small hydrophilic molecules such as glucose, at identical water content the diffusion coefficient decreases from a maximum to a minimum when going from Ia3d to Pn3m to Im3m. As a general rule, the less porous Im3m symmetry shows a slower transport efficiency at water content parity, compared with the more porous Pn3m geometry. Moreover, mesophase symmetry, lipid characteristics, and particle features must all be considered when developing systems with optimized release behavior. 12 In contrast and somewhat surprisingly, the water-channel connectivity does not influence diffusion, which is a result of the properties of the random walk in a lattice. 12
Tuning with additives
Additives can modify the structure of the lipid mesophases. For example, adding an increasing amount of hexadecane or vitamin A can tune the phase of the MO–water system. The release profile, and therefore the drug diffusion coefficient, changes with the phase. In particular, the release from the cubic phase is significantly faster than the release from the micellar, lamellar, and hexagonal phases. The geometry of self-assembled mesophases is important in determining the release rate and confirms the hypothesis that the open or closed state of the aqueous channels influences the rate of drug release.
Typical lipidic mesophases with a Pn3m, Im3m, or Ia3d symmetry are characterized by water channels with a diameter of 3–5 nm. That geometric constraint prevents large hydrophilic molecules, such as hydrophilic proteins, hormones, and antibodies, from being included in the mesophase. But that major structural limitation can be overcome by additives that increase the water-channel dimensions, including hydration-modulating agents such as sucrose stearate, phospholipids, and cholesterol. Electrostatic swelling also increases the size of water channels, either by doping lipids with charged lipids capable of swelling the mesophases or by decreasing the chemical potential of water in the nanochannels so that the total free energy of the system requires more water to equilibrate. Electrostatic swelling has been demonstrated by several research groups, 13–15 and in those studies, the cubic phases have water channels more than five times as large as traditional lipidic mesophases.
Interestingly, researchers use a combination of neutral lipids such as monopalmitolein (MP) and charged ones such as one called DSPG to design ultraswollen cubic phases of double gyroid (Ia3d), double diamond (Pn3m), and double primitive (Im3m) structures. As shown in figure
Figure 4.

Swollen lipidic mesophases transition from one structural phase to another with an increasing weight percent of water. (a) The bar graph shows how different combinations of a neutral lipid—monopalmitolein (MP)—and a charged lipid known as DSPG produce a unique range of mesophase structures. (b, c) The graphs on the right show how different percentages of DSPG and water swelling affect the structural dimension. The letters a and D stand for lattice parameter and diameter of aqueous channel, respectively. (Adapted from ref.
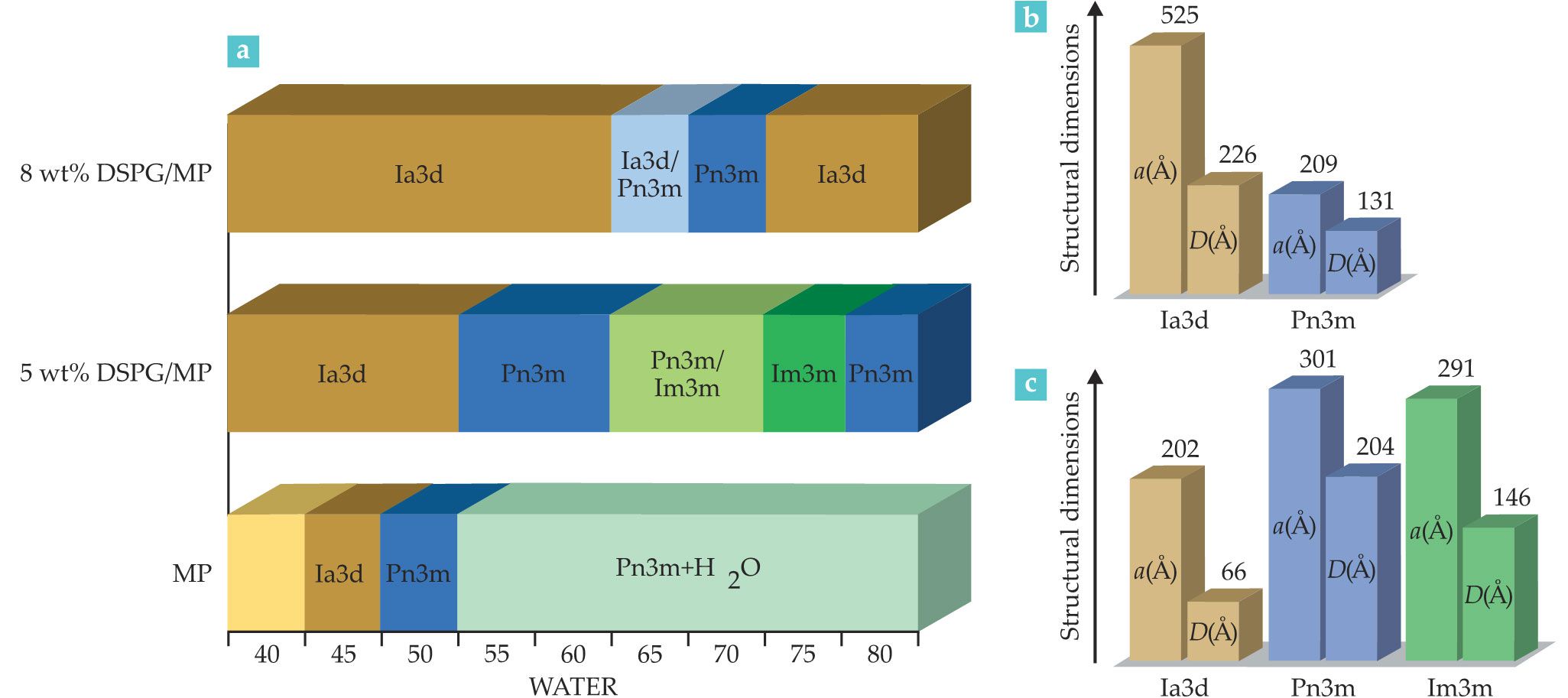
The phase reentrances indicate the competing effects at play when electrostatic swelling and increasing hydration occur together. One possible scenario is that increasing the length scales upon swelling by raising the water content decreases the effectiveness of the electrostatic interactions, which moderates the swelling effect at extreme swelling ratios. That hypothesis requires validation, and the reentrances of the phase diagram for electrostatically swollen lipidic mesophases highlight how multicomponent lipidic mesophases continue to reveal fascinating and unexpected surprises. Electrostatic swelling of the mesophase via the addition of charged lipids is a promising tool to generate thermodynamically stable swollen cubic phases. With that approach, one could load large hydrophilic biomacromolecules: The resulting lipidic surface would be decorated by a negatively charged lipid and increase the gel’s adhesion with positively charged inflamed tissues.
Adding even low concentrations of electrolytes to the aqueous phase of water–amphiphile systems can significantly modify the corresponding phase diagrams. MO–water mixtures, depending on the type and amount of electrolyte, swell and shrink the water channels. Sodium chloride or sodium sulfate dehydrates the monoolein head groups at the interface, which reduces their occupied effective area; the result is a shrinking of the water channels. On the other hand, sodium iodide leads to an increase in the hydration of the lipid head groups, expands the interfacial area occupied by a monoolein head group, and swells the water channels of the cubic phase. Low concentrations of Ca2+ can shrink the lipidic mesophase water channels, and if the Ca2+ concentration increases up to 30 millimolar, it can transform the multilamellar vesicles—formed by MO and a negatively charged lipid such as DOPG—into a cubic phase. 10
As depicted in figure
For example, figure
Figure 5.

Environmental stimuli can trigger a lipidic mesophase to release the drug cargo it’s carrying. (a) Compared with a lipid mesophase system at 40 °C, the system at 30 °C that includes vitamin A releases glucose at a faster rate. (Adapted from ref.
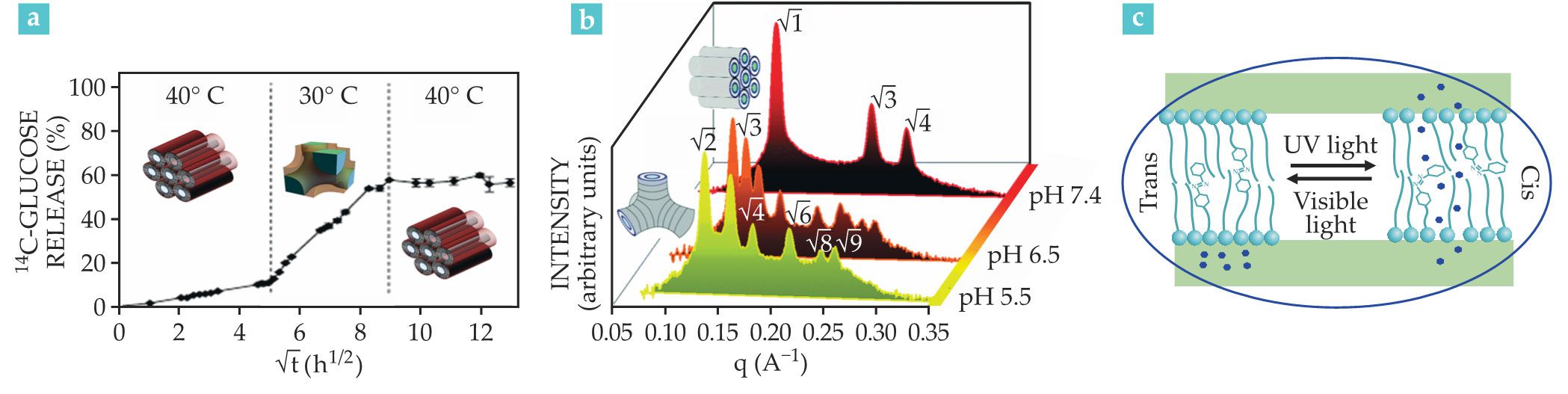
With respect to external stimuli, the application of light offers high spatial and temporal precision. Modern lasers modulate drug release profiles and provide a high level of pharmacological control by regulating the light’s wavelength, intensity, duration, and spot size. Light, therefore, can directly affect the nanocarrier’s assembly and thereby lead to drug release. As figure
The outlook for lipidic mesophases
To date, inverse bicontinuous cubic and hexagonal phases have both been shown to hold a wide range of active pharmaceutical ingredients. Because researchers can develop and design new lipid molecules capable of self-assembling into lipidic mesophases, the use of mesophases will grow quickly in the near future. Although lipidic mesophases are an interesting drug delivery system, they haven’t been as extensively commercialized as analogous products, such as liposomal delivery systems, emulsions, and nanosuspensions. To bring lipidic mesophase products to market will require a clinical translation process, and research has been more focused on gaining information about the physics of these systems. The pharmacokinetics and pharmacodynamics in the biological environment remain less advanced, and researchers are still uncertain how the kinetics of lipidic mesophase formation in the body affects tissue tolerability.

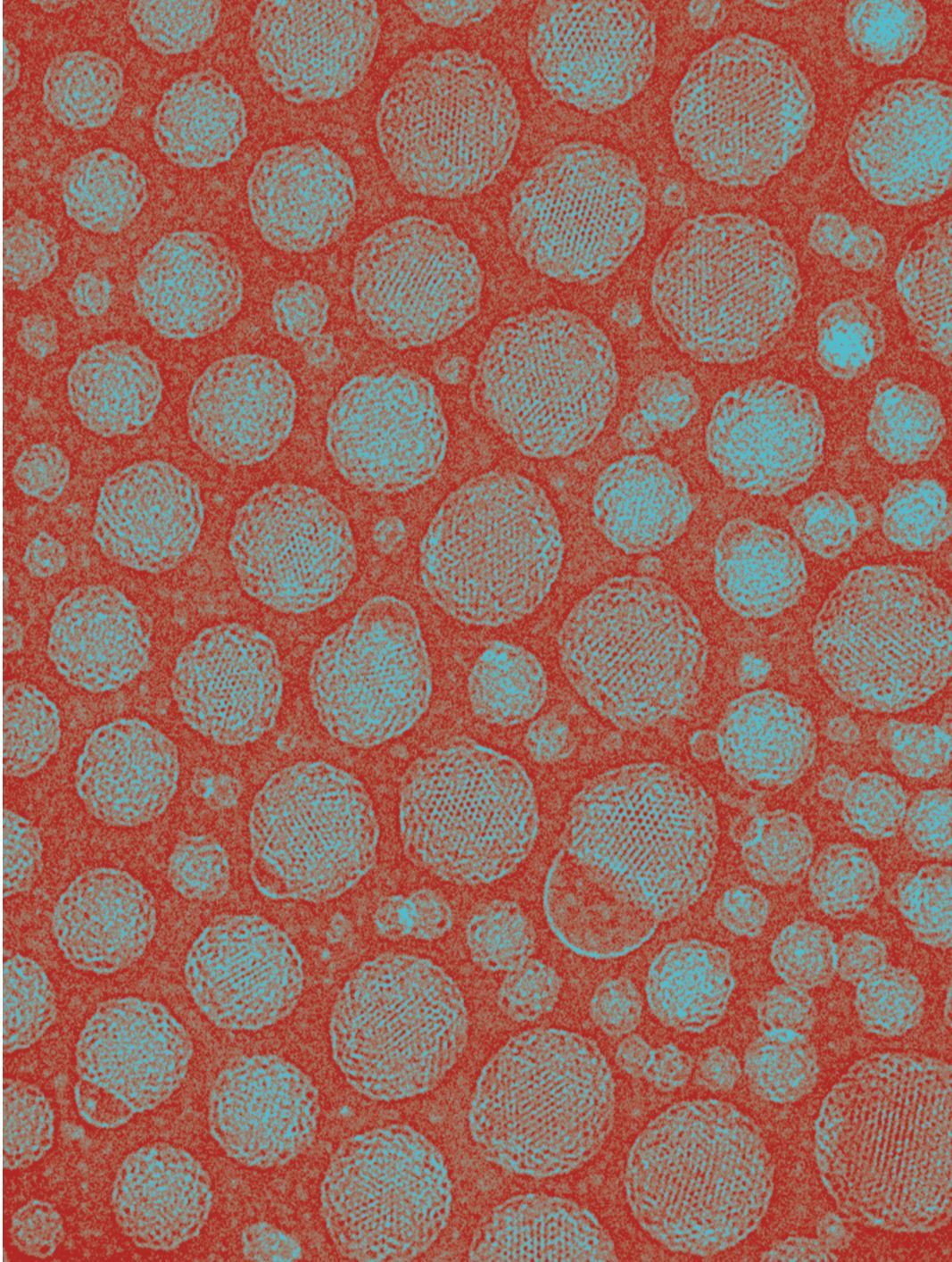
This cryogenic transmission electron microscopy image shows submicron cubosomes, formed from the cubic phase of the monolinolein lipid. (Laurent Sagalowicz, Nestlé Research Center.)
The Camurus company is developing a new technology using a lipid solution, containing the dissolved drug, that transforms into lipidic mesophases upon contact with water. The Camurus pipeline contains different liquid crystalline systems at various stages of clinical trials. For example, their FluidCrystal technology includes injection depot, topical bioadhesive, and nanoparticle formulations based on lipid self-assemblies. Once duly engineered, lipidic mesophases could outperform the more commonly used micelles, emulsions, and liposome systems in terms of local and systemic tolerability, encapsulation efficiency, physical instability, manufacturing and excipient costs, and more importantly, sustained-release control.
References
1. R. Mezzenga et al., Adv. Mater. 31, 1900818 (2019). https://doi.org/10.1002/adma.201900818
2. H. Qiu, M. Caffrey, Biomaterials 21, 223 (2000). https://doi.org/10.1016/S0142-9612(99)00126-X
3. V. Luzzati, F. Husson, J. Cell Biol. 12, 207 (1962). https://doi.org/10.1083/jcb.12.2.207
4. L. Sagalowicz et al., Trends Food Sci. Technol. 17, 204 (2006). https://doi.org/10.1016/j.tifs.2005.12.012
5. C. Fong, T. Le, C. J. Drummond, Chem. Soc. Rev. 41, 1297 (2012). https://doi.org/10.1039/C1CS15148G
6. H. M. G. Barriga, M. N. Holme, M. M. Stevens, Angew. Chem. Int. Ed. Engl. 58, 2958 (2019). https://doi.org/10.1002/anie.201804067
7. S. Aleandri et al., Langmuir 31, 12770 (2015). https://doi.org/10.1021/acs.langmuir.5b03469
8. D. Demurtas et al., Nat. Commun. 6, 8915 (2015). https://doi.org/10.1038/ncomms9915
9. R. Mezzenga et al., Langmuir 21, 3322 (2005). https://doi.org/10.1021/la046964b
10. W.-K. Fong, T. Hanley, B. J. Boyd, J. Control. Release 135, 218 (2009). https://doi.org/10.1016/j.jconrel.2009.01.009
11. I. Martiel et al., J. Control. Release 204, 78 (2015). https://doi.org/10.1016/j.jconrel.2015.02.034
12. S. Assenza, R. Mezzenga, J. Chem. Phys. 148, 054902 (2018). https://doi.org/10.1063/1.5019224
13. A. I. I. Tyler et al., Soft Matter 11, 3279 (2015). https://doi.org/10.1039/C5SM00311C
14. H. Kim, Z. Song, C. Leal, Proc. Natl. Acad. Sci. USA 114, 10834 (2017). https://doi.org/10.1073/pnas.1710774114
15. A. Zabara et al., Nat. Commun. 9, 544 (2018). https://doi.org/10.1038/s41467-018-02996-5
16. R. Negrini et al., Chem. Commun. 51, 6671 (2015). https://doi.org/10.1039/C4CC10274F
17. S. Aleandri et al., Langmuir 31, 6981 (2015). https://doi.org/10.1021/acs.langmuir.5b01945
More about the authors
Simone Aleandri is a senior pharmaceutical research scientist at the University of Bern, and Raffaele Mezzenga is a professor of food and soft materials at ETH Zürich, both in Switzerland.






