Special Issue: Water on Mars
DOI: 10.1063/1.1752425
The evolution of the Martian surface and atmosphere are increasingly being seen as connected to the behavior of water on all time scales. Over the course of a single day, even the incredibly small amounts of water in the atmosphere can form clouds that affect the energy balance at the surface, the chemistry of the atmosphere, and the geochemistry of the surface layer. On seasonal time scales, atmospheric water is part of the current climate system: It acts as a tracer of dynamical transport by the winds, and the water vapor can diffuse into (and out of) the subsurface, so that it becomes water ice at high latitudes and adsorbed water at lower latitudes. These seasonal processes, operating over many years, are thought to be responsible for large deposits of ground ice at high latitudes and for the evolution of the polar ice caps. On the million- to billion-year time scales, much larger amounts of water appear to have been present on Mars and to have played a fundamental role in sculpting the surface features that we see.
There is tremendous interest in the history and distribution of water because of the important role it plays in making a planet habitable by microorganisms and in potentially allowing life to exist. Because of the evidence for widespread liquid water on Mars throughout its history, the red planet may be the most likely place in our solar system after Earth to find evidence for present-day or past life.
The Martian climate and environment are very different from those of Earth, and the history of water cannot be predicted from first principles. Rather, we look to Mars itself to tell us what processes have been important and what role they may have played over time.
Water today
The Martian atmosphere today is predominantly carbon dioxide, with a pressure of about 6 millibars at the surface. Although the CO2 provides a slight greenhouse warming, Mars is about 1.5 times as far from the Sun as Earth and temperatures at the Martian equator average about 220 K. This is well below the freezing point of water, so we don’t expect to find stable liquid water at the surface today. 1 Spacecraft- and Earth-based telescopic measurements, however, have identified and mapped water vapor and ice clouds in the atmosphere, ice within the near-surface regions at high latitudes, and ice on the surface in the polar regions.
Planetary scientists first detected water vapor in the Martian atmosphere in 1963 using Earth-based telescopic spectroscopy. Although telescopic observations continue today and are being used to understand year-to-year variations in the water cycle, the most detailed measurements of atmospheric water were made from Viking from 1976 to 1978 and from the Mars Global Surveyor (MGS) since 1999. The measurements indicate that the atmospheric water abundance varies both with location and with season in a way that helps elucidate the processes that control it.
Michael D. Smith from NASA’s Goddard Space Flight Center in Greenbelt, Maryland, and his colleagues have analyzed the MGS observations, shown in Figure 1. 2 The average water abundances in a vertical column of the Martian atmosphere is about 20 precipitable microns—equivalent to about 2 × 10−3 g/cm2, roughly 1000 times less than the amount in Earth’s atmospheric column. The highest water abundances—about 100 precipitable µm—occur in the north polar region and are consistent with the presence of a water-ice polar deposit that is heated during summer, which then drives water into the atmosphere via sublimation. Smaller increases in water in the southern hemisphere during the southern summer suggest the release of water either from the retreating seasonal deposits of solid CO2 frost or from exposed water-ice deposits on the south polar cap. Although most of the southern residual cap retains a covering of CO2 frost at colder temperatures that preclude sublimation of any water, there appear to be at least isolated regions that lose their CO2-ice covering and reveal an underlying water-ice deposit. The atmospheric water vapor behavior at intermediate latitudes results from its transport by the winds, combined with the seasonal exchange with adsorbed water in the top few centimeters of the regolith (the global covering of loose, unconsolidated debris at the surface).

Figure 1. Seasonal and latitudinal variations in the column of water vapor in the Martian atmosphere. Units are precipitable micrometers of water, equivalent to the thickness of liquid if the vertical atmospheric column were condensed onto the surface. The horizontal axis spans three Mars years (MY); the phase of each year is marked in units of L S, the Mars-centered longitude of the Sun (where 0 indicates the first day of spring and 360 a complete Mars year). The thermal emission spectrometer on the Mars Global Surveyor made the measurements.
(Adapted from ref. 2.)
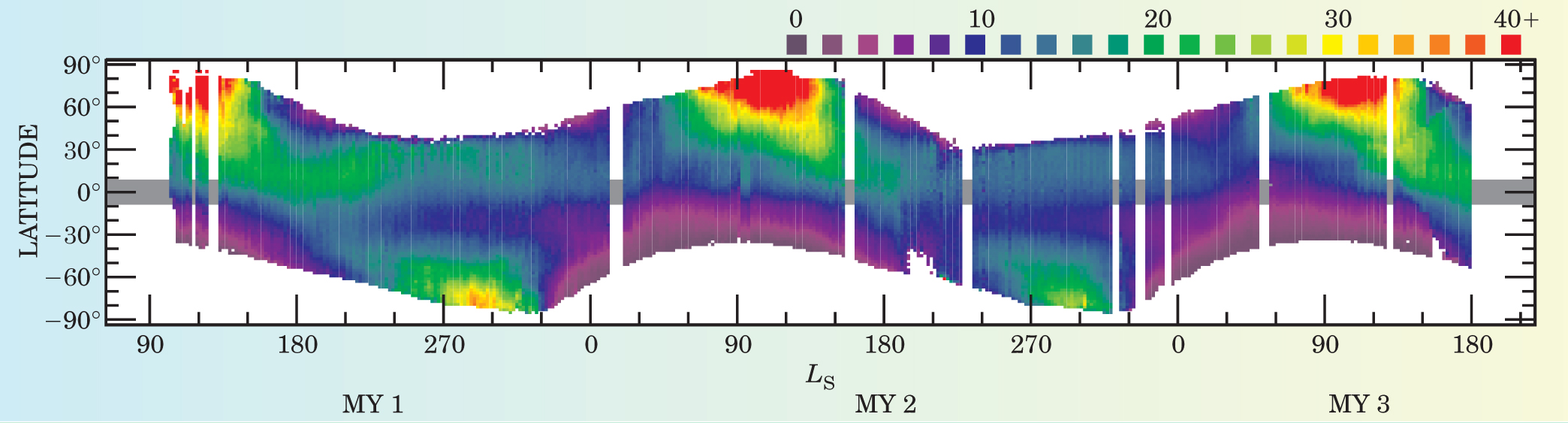
A yearly comparison of those atmospheric water vapor measurements shows that the annual cycle does not simply repeat. The water abundance during the southern summer varies by more than a factor of 10 from one year to the next. Such variation suggests that the south polar CO2-ice covering may completely disappear in some years. Were that to occur, it would expose any underlying water-ice cap, which could then heat up and sublimate water into the atmosphere. 3
Water molecules at the bottom of the atmosphere can adsorb onto individual grains in the Martian regolith. Spectroscopic measurements of sunlight reflected from the surface show a strong absorption at wavelengths at which bound water would absorb—near 3 µm—indicating that roughly 1% of the soil by mass is adsorbed water. 4 The atmospheric water vapor can also diffuse into the subsurface. At moderate and high latitudes and at depths between just 10 cm and 1 m, the subsurface temperatures are lower than the temperature at which the water vapor condenses as ice. Model calculations indicate that water can diffuse into the ground, condense to ice, and clog up the pore space in only a few tens of thousands of years. Robert Leighton and Bruce Murray of Caltech first predicted that behavior, and the two of us have modeled the expected distribution of ground ice in more detail. 5
The Mars Odyssey has a neutron spectrometer, a high-energy neutron detector, and a gamma-ray spectrometer that were able to test the models by measuring the hydrogen content of the uppermost meter or so of the subsurface. Cosmic rays strike the planet’s surface and eject high-energy neutrons from atomic nuclei. Those neutrons bounce off other nuclei, losing energy at each collision. The resulting moderate- to low-energy neutrons indicate the efficiency of the energy loss, which occurs primarily from collisions with hydrogen atoms. Thus, with a few straight-forward assumptions, the detected neutrons indicate the abundance of hydrogen and can be used to map the water content of the soil. Neutrons can also be absorbed by atomic nuclei and cause gamma-ray emission at energies characteristic of the composition; the gamma-ray energies can be used to determine abundances of elements, including hydrogen from water. The inferred geographical distribution of hydrogen matches extremely closely with the regions where stable ice is predicted to exist (see Figure 2). 6

Figure 2. Geographical variation in the water content (in percent by mass) of the uppermost meter of the Martian surface. The neutron spectrometer onboard the Mars Odyssey spacecraft measured the hydrogen content of the surface.
(Courtesy of William Feldman.)
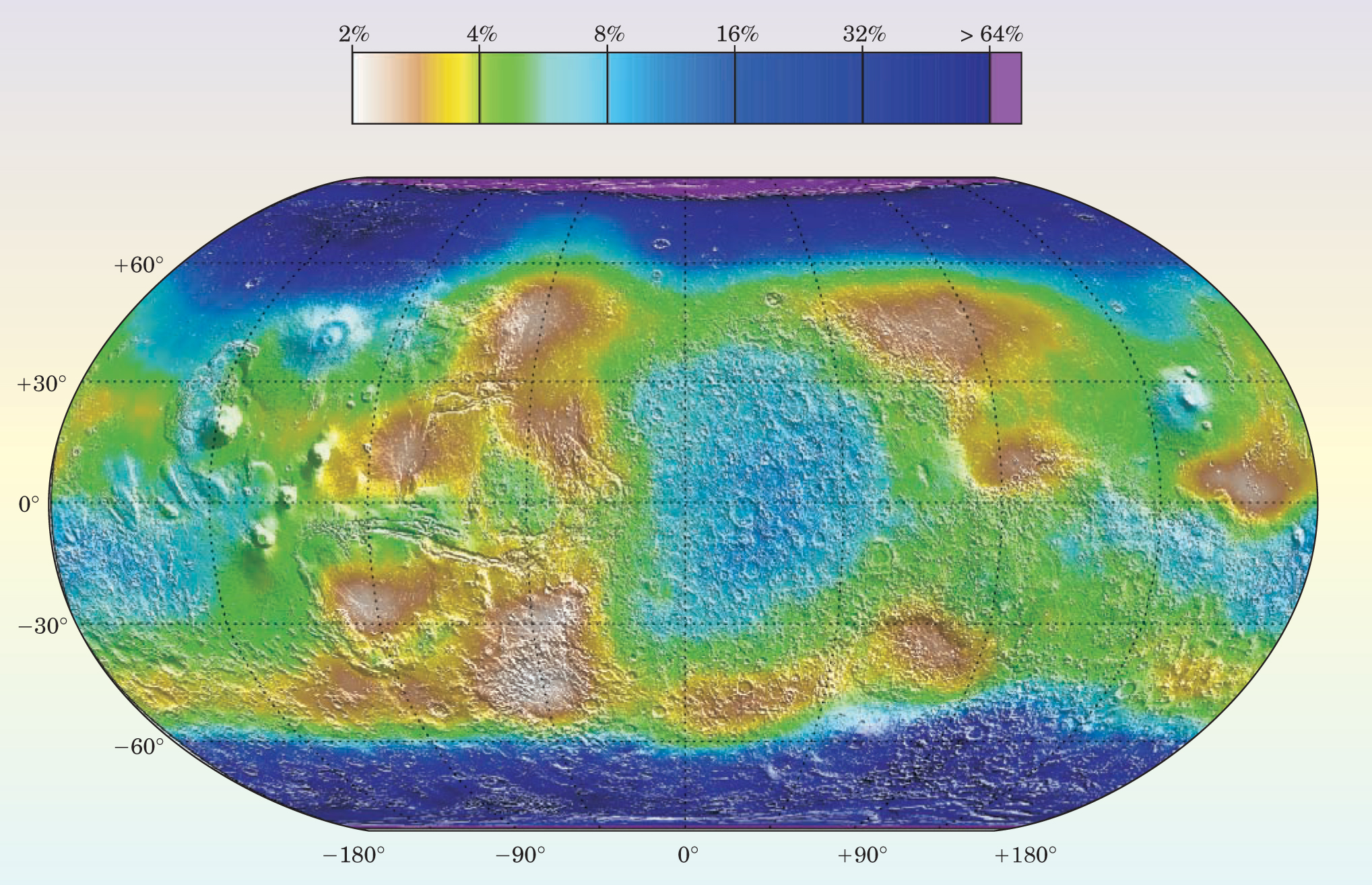
That close correspondence suggests that the models have some validity; that is, it’s likely that ice exists in regions where it can diffuse into ground from the atmosphere and form subsurface deposits. An alternative mechanism is also plausible, though. James Head, John Mustard, and their colleagues from Brown University have noted that some Martian terrain has the bumpy texture of a basketball at the several-meter scale. 7 The researchers argue that ice was deposited onto the surface along with dust during prior epochs, and that the water ice sublimated out of regions where it was not stable and remained in regions where it was stable. Although that process would create the same distribution of ground ice as the model described above, it may provide a more plausible picture for the transport of dust and ice during Martian ice ages, discussed below.
Recent water
Although ice is stable today in the top meter of the subsurface poleward of ±50–60° latitude, the surface could have been different even in the recent past. Like Earth, Mars undergoes orbital oscillations that affect its climate. A primary factor controlling the seasons and climate is the obliquity, the tilt of the planet’s spin axis with respect to the normal to the orbital plane. Today, Mars’s obliquity is 25.2°, similar to Earth’s at 23.5°. The gravitational pull from the planets—primarily Jupiter—causes that tilt to change, much like the wobble of a spinning top. Earth’s large moon stabilizes the tilt for Earth because of the large angular momentum stored in the Earth–Moon system and keeps the change in obliquity to no more than about 1.5°. But Mars, without a large moon, experiences larger oscillations. In only the past few million years, geologically recent times, Mars’s obliquity may have varied from as little as 10° to more than 40° in quasi-periodic oscillations. 8
On Earth, these oscillations are called Milankovitch cycles and can drive the terrestrial ice ages. The impact of the much larger Martian oscillations on the planet’s climate is thought to be even more significant. At high obliquity, the pole of Mars tilted more toward the Sun, and its icy polar caps experienced more direct warming by summer sunlight. Although the ice caps probably were not heated to the point of melting, they would have sublimated water ice directly into vapor. When the water vapor was carried away by the wind, it increased the global atmospheric humidity. At the same time, the soil surface cooled at lower latitudes. The combination of such effects allowed ground ice to become stable at lower latitudes than previously. Indeed, over large portions of the planet, ice probably condensed in the soil pores within the top meter of the surface. Furthermore, with enough airborne water vapor, ice also may have accumulated at the surface. Snowfall or even thick surface frosts may have blanketed the Martian landscape, particularly at higher latitudes. 9
Even a modest increase in obliquity would have dramatically changed Mars’s climate and distribution of water ice. The presence of surface and subsurface ice would have affected the geomorphic character of the landscape and left a visible record. Finding that record gives us a window into the Martian past. For example, the Martian polar caps exhibit extensive layers that have been exposed by subsequent erosion. Alternate deposits of ice and ice-rich dust or soil dominate much of the polar landscape. Layers as thin as a few meters are apparent in the highest resolution images available, and finer layers may exist that have not yet been imaged. 10 Mars’s polar caps have few impact craters to indicate any significant age, so it is widely thought that those layers represent a recent history of climate change and oscillation, probably attributable to changes in the planet’s orbit and, in particular, its obliquity.
The presence of ice-rich soil may have left a record at lower latitudes as well. In cold regions on Earth where ice-rich permafrost prevails, surface features can form by freeze–thaw cycles in the soil, movement of water in permanently frozen ground, or the emplacement and removal of ice. Some of the most pervasive features are thermal contraction polygons. As the winter cooling wave propagates into the ice-cemented soil, thermal-contraction stresses form a honeycomb network of fractures. Like their smaller cousins, mud cracks, those fractures trace out polygonal patterns typically tens of meters in scale. Unlike mud cracks, though, permafrost polygons can take thousands of years of repeated fracturing to develop a pronounced topographic signature.
Such polygonal patterns have also been observed in the highest-resolution spacecraft images of Mars (see Figure 3). These forms tend to be concentrated in the Martian high and middle latitudes, including regions down to latitudes near ±30°, slightly closer to the equator than where ground ice is expected today. A few polygons have even been found near the equator. Although some may be the result of lava cooling or desiccation of wet soil, their general appearance and latitudinal distribution is strongly consistent with an icy origin.

Figure 3. Polygonal patterned ground is ubiquitous in Earth’s terrestrial permafrost and is also commonly found on Mars, as shown here. The patterns form from seasonal thermal contraction in icy soil. Fine fractures develop into a honeycomb network that gradually fills with sand and dust over thousands of years until the pattern becomes pronounced enough to see from orbit.
(Courtesy of JPL/NASA.)

The distribution of polygons is consistent with other geological features as well. Mustard and his colleagues recently analyzed high-resolution images of an unusual surface that appears to have been mantled and then partially eroded to a few meters’ depth (see Figure 4). The “dissected” terrain in the images also occurs in the Martian mid- and high latitudes. 7 The surface appears as if it initially possessed some cohesive strength, perhaps from bonding of loose particles like sand and dust with an icy cement. As the ice disappeared, material no longer cemented together would be easily eroded by the wind and leave a partially dissected texture in the terrain. The occurrence of those features near the boundary where ice is stable and where it is not stable suggests just such a genetic relationship. The loss of ice, once stable as in-ground deposits at higher obliquity or formed from snow and windblown dust, may be responsible for the surface we see today. Thus, the surface morphology appears very suggestive of an interplay between soil and ground ice, as driven by the changing climate and the long-term actions of the same processes that operate seasonally.

Figure 4. “Dissected terrain” surfaces of Mars appear to have been mantled by a dust and ice mixture that has eroded large patches, leaving an irregular and hummocky surface beneath. This terrain generally corresponds to the edge of a region where ground ice is thought to be stable today. These features support the hypothesis that ice can sublimate from pores in the ground and leave an erodible dust surface.
(Courtesy of JPL/NASA.)

Ice has played a critical role in the evolution of the Martian landscape, but the discovery of small-scale channels and gullies by Michael Malin and Kenneth Edgett at Malin Space Science Systems in La Jolla, California, indicates the importance of liquid water as well. 11 As Figure 5 illustrates, those features, roughly 5–10 m across, are reminiscent in size and shape of water-carved gullies on terrestrial slopes. They have an appearance as though liquid water had erupted onto the Martian surface along selected slopes in the middle latitudes. Because the gullies have no impact craters on top of them, they are thought to be geologically young, perhaps only a few million years old. Yet the source of water is something of a puzzle.

Figure 5. Small-scale gullies in the middle latitudes of Mars. Water from sources yet unknown flowed downhill along valley walls and carved a series of small incised channels only 10 meters wide.
(Courtesy of JPL/NASA.)

In the current Martian climate, with a mean global temperature near 200 K, the surface layer had been thought to be permafrost down to perhaps several kilometers’ depth. But the discovery of the gullies has forced a shift in thinking. Where did this water come from? Scientists have proposed several hypotheses, ranging from the melting of ground ice or surface snow during periods of high obliquity to shallow aquifers that are preserved by an insulating blanket of soil and pressurized as they freeze. Melting ground ice or snow would require significantly different climate conditions than we see today. Freezing of a shallow aquifer would require a general cooling of the surface and subsurface. A common thread to all models, however, is that the climate must have undergone some sort of recent change. 12
Ancient water
Water played a very different, yet equally important, role on early Mars. Ancient surfaces can be identified by the number of impact craters that exist in the vicinity—the longer a surface is exposed, the greater the opportunity for asteroid impacts to excavate craters. By looking at the morphology of ancient surfaces, we can determine what geological processes acted during early epochs and what role water may have played. Although the amount of water present on Mars could be calculated from an understanding of the planet’s formation and its outgassing history, such estimates depend strongly on the assumptions. It is much safer to look to the geological evidence to determine the role of water at the surface directly. The portions of the Martian surface that date back to 3.7 billion years and older show extensive geological features that appear to have been formed by liquid water. Such features suggest that water was either more abundant or more stable at the surface then.
Two features in particular suggest the presence of liquid water. 1 Valley networks, occurring almost exclusively on the ancient surfaces, have the branching, dendritic character that is typical of valleys formed by runoff of surface water on Earth. Debate continues about whether the water that caused the erosion precipitated out of the atmosphere or was released from the crust. Either way, the dendritic character suggests long-operating processes that would work most effectively if the surface temperatures were warm enough to allow liquid water to be more stable than it is on present-day Mars.
In addition, those same ancient surfaces show evidence of erosion rates much higher than could have occurred subsequently. For example, the rims and ejecta blankets of ancient impact craters have been degraded or removed entirely over time, along with entire craters with diameters smaller than about 15 km; some craters show a spur-and-gully topography reminiscent of erosion by surface water. The higher erosion rates can be ascribed most easily to actions by liquid water on the surface and are similar to the erosion rates in some of the drier places on Earth. The higher rates suggest that water was present on Mars in abundances much greater than it is today.
How could temperatures have been warm enough nearly 4 billion years ago for liquid water to be important, especially when the Sun’s output was 30% lower than it is today? The simplest explanation is that greenhouse warming heated the surface and atmosphere sufficiently. Carbon dioxide is an efficient greenhouse gas and may have been present in abundances adequate to cause significant greenhouse warming. However, models of the warming produced by different amounts of atmospheric CO2 have problems: The atmosphere saturates with less CO2 than would have been required to raise average temperatures sufficiently to allow for liquid water, for instance. It is unlikely that the resulting CO2 clouds could hold in the heat, as they tend to mitigate the greenhouse warming. 13
Nevertheless, it isn’t clear that average temperatures need to reach 273 K by CO2 greenhouse warming alone. Additional heating can come from sunlight that penetrates a surface covering of ice (a so-called solid-state or ice greenhouse), from greenhouse warming by methane, if there were organisms capable of producing methane, or by geothermal heating from below. And, on Earth, liquid water can flow seasonally even when the average temperature is only as high as 245 K, well below the melting point of ice. 14
If a thicker CO2 greenhouse atmosphere once existed, where did all the CO2 go? It could have formed carbonate minerals by reacting with other surface minerals, been ejected into space by large impacts at the tail end of planetary accretion, or been stripped from the atmosphere by the solar wind (which was more substantial 4 billion years ago). Compelling evidence indicates that each of these processes took place. 1 An examination of meteorites on Earth that have come from Mars and the use of IR spectroscopy from orbit have revealed the presence of small amounts of carbonates on the Martian surface. Unfortunately, we have no information about the three-dimensional distribution of carbonates throughout the crust, which makes it difficult to know how much CO2 they actually incorporated. Large impact craters exist on Mars, dating from the earliest epochs, and the asteroids that created them certainly would have been able to eject atmospheric gas into space. Scientists don’t know how efficient that process was, though. And an enrichment is apparent in the heavier isotopes of the light stable elements (such as carbon, hydrogen, and nitrogen) and noble gases (such as argon and neon) in the atmosphere; we would expect that effect if gas had been lost from the atmosphere, because the heavier atoms are less readily removed and preferentially stay behind. Unfortunately, although each process appears to have acted, we don’t yet know the relative importance of each or which, if any, was the dominant loss process.
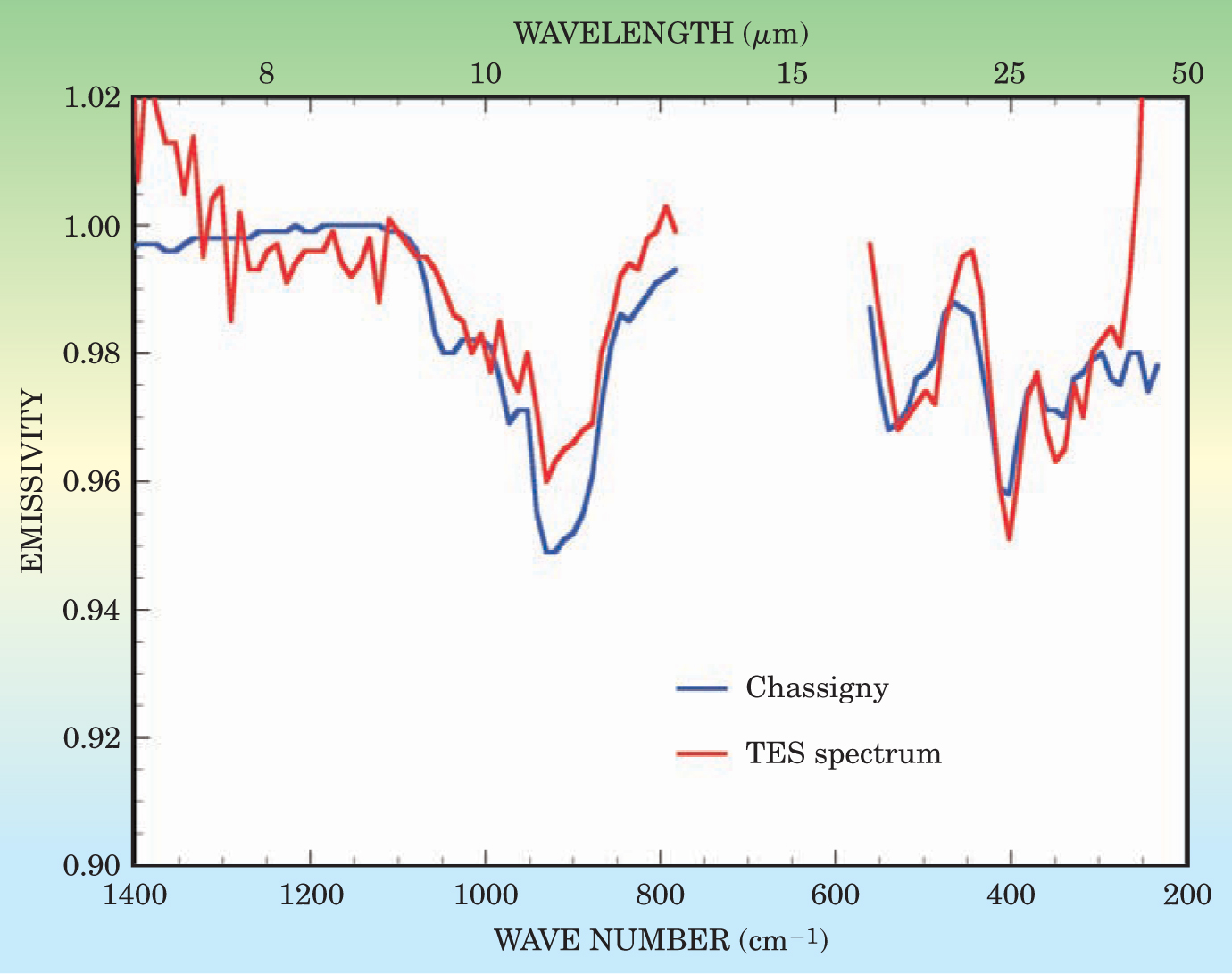
Interestingly, the apparent ubiquity of liquid water inferred from morphological evidence appears to be at odds with much of the available mineralogical data. Measurements from instruments onboard both the MGS and the Mars Odyssey show that the surface is dominated by unweathered minerals. Some of the oldest surfaces contain unaltered basalt, composed primarily of pyroxene and feldspars, and other places contain pristine olivine (see Figure 6). 15 These minerals should have reacted quickly with liquid water to form weathering products such as clays, for example. Philip Christensen of Arizona State University has argued that the presence of unaltered minerals and the absence of weathering products indicates that water has not been nearly as abundant as inferred from the geological data.

Figure 6. Comparison between a thermal emission spectrum (TES), obtained from the Mars Global Surveyor, and a laboratory spectrum of the Martian meteorite Chassigny, composed predominantly of olivine. The close correspondence of the absorption bands indicates that olivine is present in Martian surface materials.
(Adapted from ref. 15.)
One possible explanation for the discrepancy is that liquid water may have been present on Mars only during discrete episodes or isolated events in its history. In one extreme view, the water would have been mobilized by the ancient large impacts. The released water would have produced a hot torrential rain that could have carved the valley networks and eroded the surface but that didn’t last long enough to chemically alter surface minerals. 16 This hypothesis has its own problems, however, especially regarding the relative timing of impact events and erosion events typically associated with Martian climate change.
Clearly, we don’t yet understand the nature of the earliest climate on Mars and how it evolved over time. Whatever the planet’s surface climate during its earliest epochs, geochemical evidence indicates that the subsurface had abundant liquid water. We see this in the oldest of the Martian meteorites—ALH84001, for example. That meteorite contains carbonates and other minerals that would have been deposited from liquid water flowing through it nearly 4 billion years ago. And remote sensing observations indicate in a couple of places the presence of a mineral most likely formed by reactions involving crustal water. This mineral, coarse-grained hematite, can be formed by a number of processes, but the simplest explanation is that it is due to the presence of liquid water. 17
Ongoing and upcoming exploration
Many questions remain unanswered regarding the present distribution and history of water on Mars. How deep are the high-latitude ice deposits? Do subsurface aquifers exist? How extensive was liquid water in the past? NASA and the European Space Agency are operating several robotic spacecraft missions and have more planned for the future. As more data return from specialized remote sensing instruments and landed spacecraft, answers to these questions should begin to emerge.
The MGS and Mars Odyssey continue to map surface morphology and mineral and elemental abundances, in search of additional evidence for water and aqueous minerals. The European Space Agency’s Mars Express orbiter and NASA’s two Mars Exploration Rovers are in the middle of their missions to explore surface deposits in regions that may have contained liquid water. The Mars Reconnaissance Orbiter, scheduled to launch in 2005, will search for evidence of water and explore future landing sites. The first Mars Scout mission, using the Phoenix lander, will launch in late 2007. The Phoenix will land on the northern high latitudes, its goal being to access the near-surface ice for remote study of the mineralogy and chemistry.
Steven Squyres from Cornell University, principal investigator of the Mars rover missions, recently announced exciting preliminary results taken from the Mars Opportunity rover at Meridiani Planum. Earlier orbital remote sensors had detected the presence of the mineral hematite there, prompting scientists to target that site. In addition to hematite, the rover’s instruments identified the mineral jarosite and magnesium sulfate salts—all materials that require the presence of liquid water to form. The salts were in abundances of up to several tens of percent. The abundant millimeter-sized round concretions found in layered sedimentary deposits on Meridiani Planum (see Figure 7) also need liquid water to form. It is still unknown whether the water existed in the form of a lake or as subsurface liquid water and whether these mineral deposits indicate a unique or short-lived event or a longer-lived and more widespread activity of liquid water.

Figure 7. Outcrops of rock (above) located at the Mars exploration rover Opportunity landing site are only a few tens of centimeters high, but clearly show layering typical of sedimentary rock. At left is a close-up image (3 cm across) of one of the round concretions, deposits formed by precipitation of minerals around a central nucleus in the presence of abundant liquid water. Also visible are tabular-shaped holes, which suggest that mineral crystals previously present had been subsequently dissolved by the water.
(Courtesy of JPL/NASA.)

The broad goal of the missions is to better understand the Martian environment and its history, and to determine the extent to which Mars may have been able to support life at some time in its past. Researchers are hopeful that the results will provide a context in which we can understand the broader significance of either the presence or absence of life (including ancient, now extinct life). The Opportunity findings allow us, for the first time, to point to a specific location that appears to have met all of the criteria necessary to support life. The much more detailed analyses necessary to determine whether there was ever life on Mars will require either a new generation of analytical capabilities on a more sophisticated Mars rover or the return of samples back to Earth for detailed study.
But with five active spacecraft at Mars, two more under construction, and several in detailed planning, planetary scientists are in the middle of a vigorous and exciting period of Mars exploration. The questions that we are asking about the history of the geology, the climate, and possibly life are exciting questions, and we now have the tools to answer them. Whatever answers emerge will address fundamental questions about the nature of planets, their evolution, and the potential distribution of life in the universe.
References
1. B. M. Jakosky, R. J. Phillips, Nature 412, 237 (2001).https://doi.org/10.1038/35084184
2. M. D. Smith, J. Geophys. Res. 107, 5115 (2002).https://doi.org/10.1029/2001JE001522
3. M. I. Richardson, R. J. Wilson, J. Geophys. Res. 107, 5031 (2002).https://doi.org/10.1029/2001JE001536
4. L. A. Soderblom, in Mars, H. H. Kieffer, B. M. Jakosky, C. W. Snyder, M. S. Matthews, eds., U. of Ariz. Press, Tucson (1992), p. 557.
5. R. B. Leighton, B. C. Murray, Science 153, 136 (1966);https://doi.org/10.1126/science.153.3732.136
M. T. Mellon, B. M. Jakosky, J. Geophys. Res. 100, 11781 (1995);https://doi.org/10.1029/95JE01027
M. T. Mellon, W. C. Feldman, T. H. Prettyman,Icarus (in press).6. W. V. Boynton et al., Science 297, 81 (2002).https://doi.org/10.1126/science.1073722
7. J. F. Mustard, C. D. Cooper, J. F. Rifkin, Nature 412, 411 (2001);https://doi.org/10.1038/35086515
J. Head et al., Nature 426, 797 (2003).https://doi.org/10.1038/nature021148. J. Laskar, P. Robutel, Nature 361, 608 (1993).https://doi.org/10.1038/361608a0
9. B. M. Jakosky, M. H. Carr, Nature 315, 559 (1985);https://doi.org/10.1038/315559a0
M. A. Mischna et al., J. Geophys. Res. 108, 5062 (2003).https://doi.org/10.1029/2003JE00205110. M. C. Malin et al., Science 279, 1681 (1998).https://doi.org/10.1126/science.279.5357.1681
11. M. C. Malin, K. S. Edgett, Science 288, 2330 (2000).https://doi.org/10.1126/science.288.5475.2330
12. M. T. Mellon, R. J. Phillips, J. Geophys. Res. 106, 23165 (2001);https://doi.org/10.1029/2000JE001424
P. R. Christensen, Nature 422, 45 (2003).https://doi.org/10.1038/nature0143613. F. Forget, R. T. Pierrehumbert, Science 278, 1273 (1997).https://doi.org/10.1126/science.278.5341.1273
14. C. P. McKay et al., Nature 313, 561 (1985).https://doi.org/10.1038/313561a0
15. V. E. Hamilton et al., Meteoritics Planet. Sci. 38, 871 (2003).https://doi.org/10.1111/j.1945-5100.2003.tb00284.x
16. T. L. Segura et al., Science 298, 1977 (2002).https://doi.org/10.1126/science.1073586
17. P. R. Christensen et. al., J. Geophys. Res. 105, 9623 (2000).https://doi.org/10.1029/1999JE001093
More about the authors
Bruce M. Jakosky, (bruce.jakosky@lasp.colorado.edu) Laboratory for Atmospheric and Space Physics, University of Colorado, Boulder, US .
Michael T. Mellon, (bruce.jakosky@lasp.colorado.edu) Laboratory for Atmospheric and Space Physics, University of Colorado, Boulder, US .




