Lanthanide-doped upconversion nanoparticles
DOI: 10.1063/PT.3.2913
Photon upconversion—a light–matter interaction that turns low-energy laser stimulation into high-energy luminescent emissions—is one of the most fascinating effects in nonlinear optics. Much of what we know about the phenomenon comes from work—particularly the groundwork of Peter Franken and colleagues at the University of Michigan 1 —using the newly invented lasers of the early 1960s, which provided the required high-intensity coherent light source. By focusing a ruby laser beam into a quartz crystal, Franken and colleagues doubled the light’s frequency. That phenomenon, now known as second-harmonic generation, arises from an interaction between the electric field and its spatial derivative.
Shortly after the publication of Franken’s paper, Wolfgang Kaiser and Charles Garrett at Bell Labs observed a different upconversion process, two-photon excitation fluorescence, in a europium-doped crystal irradiated by an intense laser beam. 2 In contrast to second-harmonic generation, two-photon absorption requires the incident photons to be absorbed simultaneously to accumulate the energy for photon upconversion. Both techniques have inherent limitations: Their implementation requires either a costly high-power pulsed laser or an upconversion medium without inversion symmetry.
A conceptual revolution came with the discovery that dopants from the lanthanide series of elements provide intermediate electronic states that can store one low-energy photon’s energy until a second one arrives. Thanks to their partially forbidden electronic transitions, lanthanide ions’ excited states have lifetimes of up to several milliseconds. As a result, lanthanide-doped materials enable photon upconversion to proceed through sequential absorption of two or more low-energy photons even with a low-power continuous-wave laser.
The modern notion of lanthanide-based photon upconversion dates back to 1961, when John Porter observed luminescence in a crystal of praseodymium-doped lanthanum chloride (LaCl3:Pr) upon exciting the Pr3+ ions with nonsimultaneous photons. 3 In the years that followed, as shown in figure 1, several researchers contributed to the development of lanthanide-sensitized upconversion, which makes use of energy transfer between dopant ions. First came the landmark experiment of François Auzel in 1966: In a glass codoped with ytterbium and either erbium or thulium, low-energy photons were absorbed by the Yb3+ ions, and higher-energy photons were emitted by the Er3+ or Tm3+. Another notable result was the observation of photon avalanche, which involves the transfer of energy between identical dopant ions, in LaCl3:Pr by Jay Chivian and colleagues at Vought Corporation Advanced Technology Center in 1979.
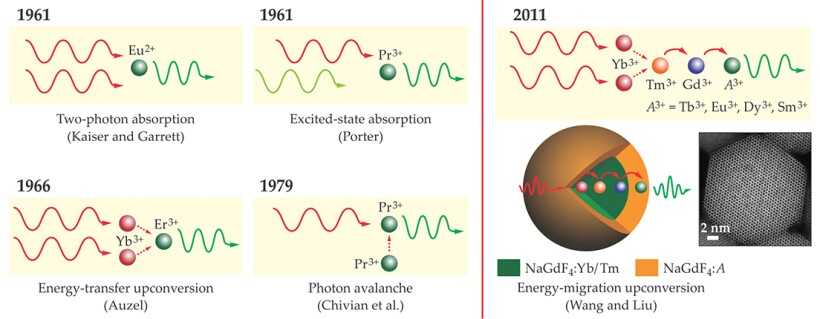
Figure 1. The historical development of photon upconversion in lanthanide-doped bulk materials dates back to the early 1960s. Wolfgang Kaiser and Charles Garrett observed two-photon absorption in a europium-doped crystal, but the photons had to arrive simultaneously. John Porter demonstrated excited-state absorption using nonsimultaneous photons. François Auzel and Jay Chivian and colleagues developed upconversion processes based on energy transfer from one lanthanide ion to another. In 2011 Feng Wang and one of us (Liu) developed core–shell nanoparticles in which energy migrates outward from one lanthanide (ytterbium in this case) to another (the activator A) through a series of steps. (Nanoparticle image adapted from ref.
Until the beginning of the 21st century, however, photon upconversion was investigated primarily in bulk glasses or crystalline materials, and much of its promise was left untapped. That situation changed dramatically with the development of upconversion nanomaterials. At the nano level, many new effects start to come into play that open up new possibilities for upconversion.
For example, the fundamental challenge in the early days of investigation was that an efficient upconversion material needs a ladder-like arrangement of energy levels to absorb the incident laser energy in equal increments. For that reason, early work on upconversion focused almost exclusively on the few dopants that meet that stringent requirement—namely, Er3+, Tm3+, and holmium ions—and the accessible range of emission wavelengths was thus severely restricted. An important breakthrough came in 2011 with the development of a core–shell nanostructure with a different dopant in each layer. In the structure shown in figure 1, the core dopant, called the sensitizer, absorbs the incident laser photons. The energy is then transferred through a series of steps to the shell dopant, or activator, which emits the upconverted radiation. For the most part, Yb3+ and neodymium are the materials of choice for the sensitizer because of their large absorption cross sections, Yb3+ at 980 nm and Nd3+ at 800 nm. But the activator can be any of a number of lanthanides, without the need for ladder-like energy levels. 4 Core–shell upconversion nanoparticles can therefore be made to emit in any color. With the emergence of nanotechnology, photon upconversion techniques are well positioned for numerous applications in modern technology.
Biomedical possibilities
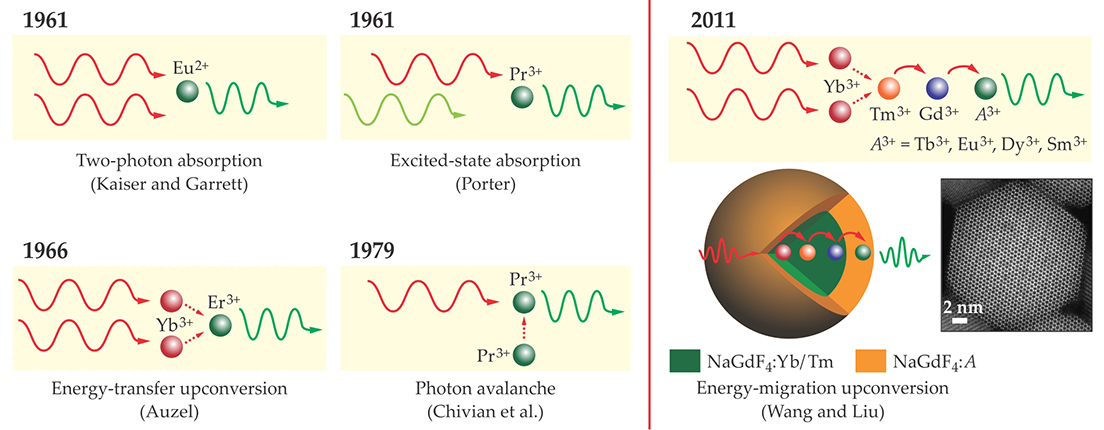
Although the research on lanthanide-doped upconversion nanoparticles is still in its infancy, impressive results are already emerging. Researchers are targeting a wide range of potential applications, including single-particle tracking, bioimaging and therapeutics, thermal sensing, photovoltaics, anticounterfeiting, and full-color volumetric three-dimensional displays.
The ability to use luminescence to track individual nanoparticles in real time inside a living organism (see figure 2) could enable revolutionary advances in the biological and medical sciences. For example, such a technique could provide important information about the uptake pathway and distribution of pharmaceutical nanoparticles injected into a patient. (For more on nanoparticles in cancer therapy, see the article by Jennifer Grossman and Scott McNeil, Physics Today, August 2012, page 38

Figure 2. Biological and medical applications of lanthanide-doped upconversion nanoparticles include sensing, imaging, and photodynamic therapy (described in the text). Functionalizing the particles’ surfaces with small molecules, oligonucleotides, proteins, or peptides improves their dispersibility, wettability, and biocompatibility. (Adapted from ref.
Furthermore, compared with the UV and blue light used to excite quantum dots, the near-IR light that excites upconversion nanoparticles penetrates tissue to a greater depth (more than 1 cm), does less photodamage to cells, and doesn’t induce autofluorescence, the natural emission of light by biological structures excited by UV or visible light. Upconversion nanoparticles themselves exhibit high photochemical stability and low cytotoxicity.
A problem that arises in biomedical applications is the loss of energy from excited activator ions to so-called surface quenchers in the surrounding medium. In a nanoscale particle, a large percentage of activator ions are exposed at the particle surface, so surface quenching can rapidly deplete their excitation energy. 7 To minimize that energy loss, a popular approach is to passivate the nanoparticles with an inert shell that separates the dopants from the surface quenchers. 8 The emission intensity of passivated nanoparticles is typically one to two orders of magnitude higher than that of their nonpassivated counterparts. In fact, the shell needn’t be completely inert: It can be doped with additional sensitizer ions to enhance the upconversion performance or with magnetic or radioactive lanthanide ions to enable multimodal imaging.
To make upconversion nanoparticles not only emissive but also biocompatible, their surfaces can be functionalized with a broad range of ligands such as small molecules, dendrimers, polymers, and biomolecules. Surface functionality imparts to the nanoparticles the selectivity or specific recognition required for biosensing. For instance, by conjugating upconversion nanoparticles with molecular energy acceptors, one can perform fluorescence resonance energy transfer sensing, in vivo and at high sensitivity, of intracellular metal ions such as zinc and mercury and bioactive molecules such as glutathione and avidin.
Another important area for applications of upconversion nanoparticle conjugates is their use for photodynamic therapy. Cancer cells are vulnerable to certain photosensitive chemicals, such as zinc(II) phthalocyanine, excited with red light. But red light doesn’t penetrate tissue well. Instead, the photosensitizers can be attached to a red-emitting particle’s surface, transported to the tumor, and excited with near-IR light. That approach offers the ability to treat tumors that extend farther beneath the skin surface.
One principal drawback of using upconversion nanoparticles for biomedical applications is the strong absorption of water molecules at 980 nm, one of the two available excitation wavelengths. Prolonged excitation at that wavelength inevitably causes the surrounding solution to overheat. The use of Nd3+-sensitized nanoparticles, excited at 800 nm, could minimize the overheating issues by tremendously reducing water absorption. 9
Other applications
Many of the lanthanides’ photophysical properties are temperature dependent, including the emission intensity and the excited-state lifetime or rise time. That temperature sensitivity raises the exciting possibility of using upconversion nanoparticles for high-resolution contactless thermal sensing, even in harsh environments and under the influence of high electromagnetic fields. 10 The implications of upconversion nanothermometry are at least as significant as those of upconversion bioimaging. But an important criterion must be met—namely, thermal equilibrium in the population of excited states. Nanoparticles codoped with Yb3+ and Er3+ and excited at 980 nm are well suited to that requirement. Because they are excited by a multiphoton process, the spatial resolution of the measurement could be much higher than that attainable by one-photon excited nanothermometers. However, the use of Yb3+/Er3+-codoped nanoparticles for in vivo temperature measurements is challenging due to the weak transmission of upconversion emissions at 520 nm and 540 nm through animal tissues.
Solar-cell efficiencies can, in principle, be dramatically improved by using upconversion nanoparticles to harvest abundant near-IR radiation, which normally goes to waste, and convert it into photons with energies above the cell’s semiconductor bandgap. (For more on solar energy conversion, see the article by George Crabtree and Nathan Lewis, Physics Today, March 2007, page 37
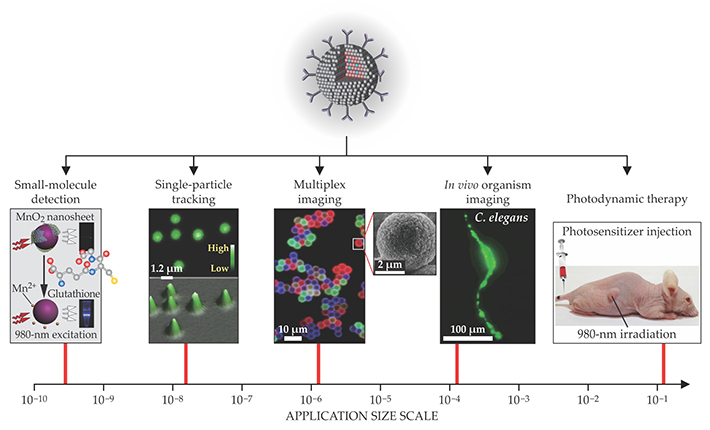
Because upconversion nanoparticles can be readily deposited or printed as films from solution, they can be used for security applications such as anticounterfeiting. Shining a beam of near-IR light over the printed area reveals a hidden pattern that is almost impossible to counterfeit. Different types of nanoparticles can be combined to create multicolor barcodes 13 and other security patterns, as shown in figure 3. In addition, the luminescence lifetime of the lanthanide ions, which can be tuned from microseconds to milliseconds by varying their doping concentration, can also be used to carry information. 14 That feature has rarely been explored in other luminescent materials.
Figure 3. Three-dimensional displays and security technologies are some of the emerging applications motivating the development of upconversion nanocrystals. Because both the emission color and the excited-state lifetime of the particles are tunable, both can be used to store information in bar codes and other security patterns. (Adapted from refs.
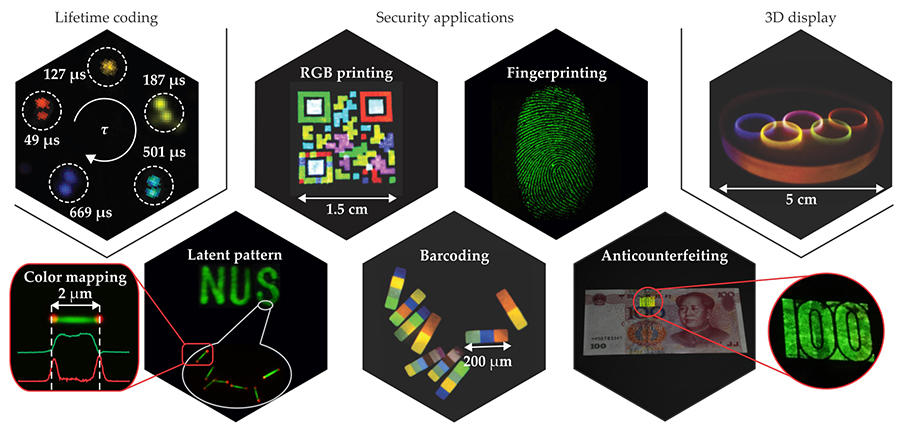
Photon upconversion provides a promising alternative to existing color display technologies. It offers many advantages, including high brightness, a wide color gamut, highly saturated emission colors—and the potential for volumetric 3D displays. 15 The 3D display technology is enabled by the nonlinear optical properties of upconversion nanoparticles embedded in a solid transparent matrix. Excitation light above a certain power density threshold—around 1 W/cm2—is typically needed to initiate upconversion. A focused near-IR beam can therefore selectively excite particles at its focal point where the power density is highest. By rapidly scanning the laser, one can illuminate the entire display with sharp contrast, high brightness, and ultrahigh resolution. With optical components that are relatively cheap and simple to put together, the display has the potential to outperform currently available holographic technologies and glasses-based stereoscopic displays. The ability to remotely tune the emission color of the nanoparticles may support a wide range of applications.
Particle design
To synthesize upconversion nanoparticles, one must carefully choose both the dopants and the host materials that accommodate them. The lanthanide ions, typically existing in their most stable, trivalent oxidation state, gain their optical properties from transitions between their 4f subshell electrons buried in the interior of the atoms. The coulombic interactions and spin–orbit coupling of those electrons lead to abundant energy sublevels and a large number of emitting levels.
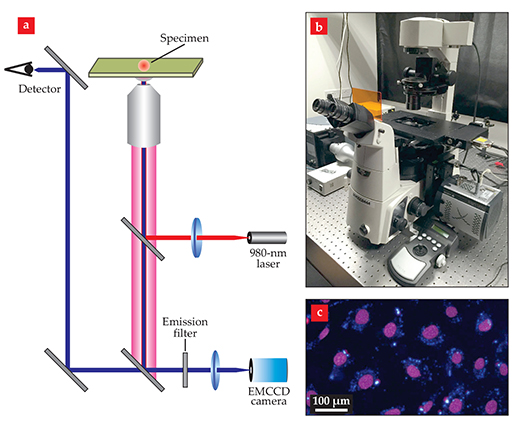
The visualization of intense upconversion emission from nanoparticles, either dispersed in solution or in solid form, means that an efficient energy transfer has happened from the sensitizer to the activator. For photon upconversion to proceed, the energy released through deactivation of the excited sensitizer needs to be matched with the energy gap between two energy levels of the activator. Only then can nonradiative energy transfer be more rapid than radiative deactivation. A basic optical microscope setup useful in visualizing the upconversion emission of the nanoparticles is pictured in figure 4.

Figure 4. An optical microscope system for characterizing upconversion nanoparticles. (a) A continuous-wave 980-nm laser excites the particles, and a detector and electron-multiplying (EM) CCD camera use the upconverted light to image the specimen. (b) The microscope, shown here, can be integrated with a spectrometer to allow luminescence imaging and spectroscopy to be performed simultaneously. (c) This luminescence image shows cells stained with upconversion nanoparticles that emit blue light. The purple color of the cell nuclei comes from an organic dye.
Because electronic transitions between f orbitals are forbidden by symmetry, the emission spectra of isolated lanthanide ions are rather weak. However, the transitions may become partially allowed through interaction between the ions and a host lattice. That interaction stems from the crystal field induced by the host material around the dopant ions. A remarkable body of accrued evidence suggests that the crystal field’s effect on a lanthanide’s electronic transitions is highly sensitive to the ion’s site symmetry: A more asymmetric environment tends to produce a stronger crystal field. For example, hexagonal-phase NaYF4 nanoparticles typically offer about an order-of-magnitude enhancement of upconversion efficiency relative to their cubic-phase counterparts. Site symmetry can be broken—and luminescence enhanced—by doping the host with small ions such as lithium: The lattice strain due to the size mismatch between the dopant and host cations can appreciably distort the site symmetry and hence alter the crystal field.
Importantly, host materials can exchange energy with the lanthanide dopants. The short distance between host ions and lanthanide dopants leads to a high probability of nonradiative host ion–dopant interactions, and, consequently, a portion of the energy carried by the lanthanides is absorbed by the host through vibrational coupling. Unfortunately, direct energy exchange between the host lattices and lanthanide dopants can be detrimental to upconversion. To minimize such nonradiative energy loss and maximize radiative emission, it is vital to use host materials with low lattice phonon energies. Fluorides are excellent candidates because of their low phonon energies and high chemical stability.
Dopant–dopant interactions, which are linked to the distance between dopant ions and thus to the doping concentration, also affect photon upconversion. One might guess that a large amount of Yb3+ sensitizer should result in a high upconversion efficiency, thanks to the Yb3+ ion’s high absorption cross section at 980 nm. However, at Yb3+ concentrations above 20 mol%, luminescence is considerably quenched, particularly at shorter wavelengths. The quenching is most likely caused by an increased probability of energy migration through Yb3+ ions to lattice defects or surface quenchers. A high Yb3+ concentration may also result in an increased probability of back-energy transfer from activator to sensitizer, rather than from sensitizer to activator.
Specially designed host-crystal structures could prevent the migration of excitation energy to defects, even at high Yb3+ concentration. Energy migration depends more on the distance between Yb3+ ions than on dopant concentration, so arranging the Yb3+ ions into discrete clusters separated by large distances could solve the problem. Although such a structure is theoretically possible, achieving it in practice has been fraught with synthetic difficulties. It was only recently that orthorhombic Er3+-doped potassium ytterbium fluoride nanoparticles with a tetrad-cluster arrangement of Yb3+ proved effective in preserving excitation energy. Those particles produced an unusual emission at 410 nm, which could enable light-triggered biological reactions and photodynamic therapy in need of light activation in the violet regime.
Although activators are used at lower concentrations than sensitizers, their interactions still must be carefully managed to account for several nonradiative deactivation pathways that can substantially decrease UV or visible emission intensity. For instance, the blue emission at 475 nm of NaYF4:Yb/Tm nanoparticles drops markedly when the doping concentration of Tm3+ is raised from 0.2 mol% to 2.0 mol%; the decrease can be ascribed to the greater probability of cross-relaxation, or energy transfer between two Tm3+ ions.
Cross-relaxation between different types of activators can also occur if their optical transitions between specific energy levels match well with each other. Such nonradiative deactivation makes it challenging to combine certain pairs of activators in a single nanoparticle. One effective solution for suppressing that cross-relaxation is to adopt a core–shell design with different activators segregated into separate layers. The development of core–shell nanoparticles with multiple activators has greatly expanded the range of available upconversion emission profiles.
In some cases, cross-relaxation can be used beneficially to modulate the intensity ratios of different emission peaks. For example, precisely designed cross-relaxation between Ho3+ and cerium activators in NaYF4:Yb/Ho/Ce nanoparticles can selectively quench the green emission bands of Ho3+ and considerably enhance red emission intensity at 674 nm.
Outlook
Formidable challenges still stand in the way of the promise of upconversion nanoparticles. 16 One already mentioned is the problem of low upconversion quantum efficiency. To date, the most efficient activators—Er3+, Tm3+, and Ho3+—offer maximum upconversion efficiencies of less than 10%. Developing new particle systems with broadband absorption and higher efficiency is important for the field to advance.
Using transition-metal ions as codopants can also promote photon upconversion. Not only do they offer much greater compositional flexibility, but they can also enable broadband emission, which is not a typical property of most lanthanide ions. For example, broad green upconversion emission has been reported at room temperature in lanthanum magnesium hexaaluminate phosphors doped with manganese and Yb3+ ions. 17 That behavior was attributed to the formation of Yb3+–Mn2+ dimers, 18 whose energy levels are sketched in figure 5a. Due to the strong crystal field around them, the transition-metal ions can provide broad and host-sensitized excitation or emission bands.

Figure 5. At the frontiers of photon upconversion, researchers seek to extend the versatility of lanthanide-doped nanoparticles through transition-metal codoping or modulated pulse excitation. (a) Codoping nanoparticles with ytterbium and the transition metal manganese leads to the formation of Yb3+–Mn2+ dimers with a rich set of energy levels, some of which are sketched here. Upconversion can proceed through either excited-state absorption or energy-transfer processes. The resulting upconversion emission spectrum is much broader than can usually be obtained using lanthanides alone. (b) In a nanoparticle triply doped with Yb3+, cerium, and holmium, a 6-ms excitation pulse at 980 nm facilitates the accumulation of population in a relatively low excited state of Ho3+, due to cross-relaxation between Ce3+ and Ho3+, and enhances the red emission at 646 nm. In contrast, a 0.2-ms pulse suppresses the excitation of Ce3+, allows Ho3+ to be promoted to higher excited states, and gives rise to strong emission at 541 nm.
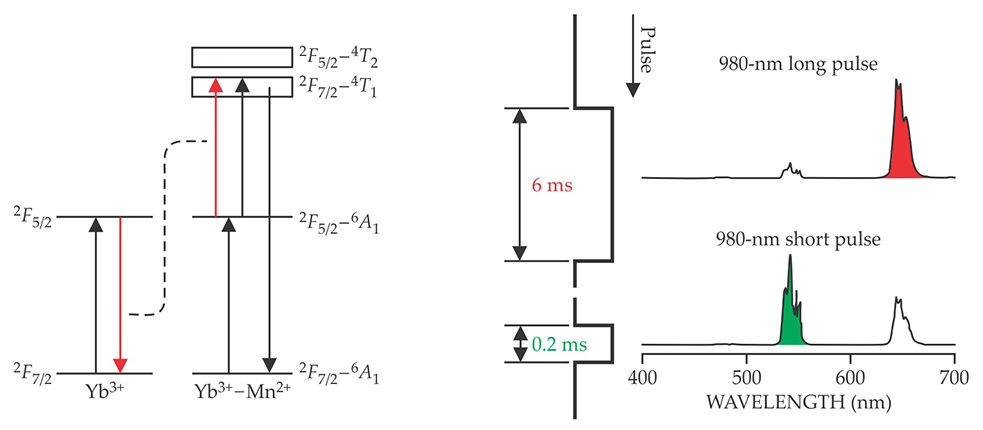
A promising but challenging frontier in the field of upconversion is controlling the color output by external measures such as pulsed excitation. Figure 5b shows an example of a system whose emission can be switched between red and green, depending on the duration of the excitation pulse. Such nanomaterials could find application in multicolor displays or anticounterfeiting systems.
We are inspired by recent results and claims of improved efficiency, but more effort is needed to develop robust tests and verifications. Time-resolved spectroscopic experiments on single upconversion nanoparticles, either on a dry substrate or under physiological conditions, will be rewarding, but their analysis will be challenging, because the integration of existing imaging systems must meet exceedingly high standards of performance and reliability. A cooperative and coordinated effort across a broad spectrum of disciplines can help to make upconversion nanoparticles viable for large-scale practical applications.

(Adapted from ref.

References
1. P. A. Franken et al., Phys. Rev. Lett. 7, 118 (1961). https://doi.org/10.1103/PhysRevLett.7.118
2. W. Kaiser, C. G. B. Garrett, Phys. Rev. Lett. 7, 229 (1961). https://doi.org/10.1103/PhysRevLett.7.229
3. F. Auzel, Chem. Rev. 104, 139 (2004). https://doi.org/10.1021/cr020357g
4. F. Wang et al., Nat. Mater. 10, 968 (2011). https://doi.org/10.1038/nmat3149
5. S. Wu et al., Proc. Natl. Acad. Sci. USA 106, 10917 (2009). https://doi.org/10.1073/pnas.0904792106
6. J. Zhao et al., Nat. Nanotechnol. 8, 729 (2013). https://doi.org/10.1038/nnano.2013.171
7. D. R. Gamelin, H. U. Güdel, in Transition Metal and Rare Earth Compounds: Excited States, Transitions, Interactions II, H. Yersin, ed., Springer (2001), p. 1.
8. S. Han et al., Angew. Chem. Int. Ed. 53, 11702 (2014). https://doi.org/10.1002/anie.201403408
9. Y.-F. Wang et al., ACS Nano 7, 7200 (2013). https://doi.org/10.1021/nn402601d
10. C. D. S. Brites et al., Nanoscale 4, 4799 (2012); https://doi.org/10.1039/c2nr30663h
C. D. S. Brites et al., Adv. Mater. 22, 4499 (2010). https://doi.org/10.1002/adma.20100178011. D. Chen et al., Chem. Commun. 48, 5898 (2012). https://doi.org/10.1039/c2cc32102e
12. W. Zou et al., Nat. Photonics 6, 560 (2012). https://doi.org/10.1038/nphoton.2012.158
13. J. Lee et al., Nat. Mater. 13, 524 (2014). https://doi.org/10.1038/nmat3938
14. Y. Lu et al., Nat. Photonics 8, 32 (2014). https://doi.org/10.1038/nphoton.2013.322
15. R. Deng et al., Nat. Nanotechnol. 10, 237 (2015). https://doi.org/10.1038/nnano.2014.317
16. X. Liu, C.-H. Yan, J. A. Capobianco, Chem. Soc. Rev. 44, 1299 (2015). https://doi.org/10.1039/C5CS90009C
17. R. Martín-Rodríguez, R. Valiente, M. Bettinelli, Appl. Phys. Lett. 95, 091913 (2009). https://doi.org/10.1063/1.3220059
18. R. Martín-Rodríguez et al., Phys. Rev. B 82, 075117 (2010). https://doi.org/10.1103/PhysRevB.82.075117
More about the authors
Marco Bettinelli is a professor in the department of biotechnology at the University of Verona in Verona, Italy. Luís Carlos is a professor in the department of physics and the Centre for Research in Ceramics and Composite Materials at the University of Aveiro in Aveiro, Portugal. Xiaogang Liu is a professor in the department of chemistry at the National University of Singapore and a senior scientist at the Institute of Materials Research and Engineering of the Agency for Science, Technology, and Research, also in Singapore.







