Frontiers of surface science
DOI: 10.1063/1.2800097
To many physicists, “surface science” means the study of clean crystalline surfaces under ultrahigh vacuum. That narrowly defined field has yielded some interesting results, such as the reconstruction of the structure of surfaces and the recognition that atomic defects are chemically active sites. But a host of new systems and new phenomena are introduced, with many important applications, by so-called buried interfaces: solid surfaces in contact with gases, liquids, or even other solids. Interfaces between solids and aqueous or other solutions turn up in electrochemistry and in biophysics—many such interfaces are found in the human body.
Of particular interest to the two of us, and a focus of this article, is the catalytic behavior a surface can exhibit when in contact with a gas. A molecule adsorbed onto a surface forms chemical bonds with the surface atoms; those bonds change the electronic structure and arrangements of both the molecule and the surface atoms. If the change in structure lowers the energy barrier to a chemical reaction without damaging the surface, then the surface acts as a catalyst.
Nanoparticles, too, can act as catalysts. With the rise of nanosciences, one can now synthesize monolayers of nanoparticles with controlled size and shape, which allows for greater control over the reaction output. That is a uniquely important development because much of 21st-century catalysis science so far focuses on reaction selectivity, or the production of one type of molecule out of several different but thermodynamically feasible products. 1 In the developing field of green chemistry, reaction selectivity is needed to eliminate undesirable byproducts—waste—in chemical or energy conversion processes. 2 The size, composition, and surface structure of the catalyst nanoparticles are important ingredients that control reaction selectivity on the atomic scale.
Concepts uncovered by vacuum surface science
Surface science initially evolved as the study of surfaces under vacuum because so many of the techniques developed to look at surfaces can only operate under vacuum. Such techniques involve probing the surface with a beam of electrons, atoms, molecules, or ions, or—in the case of x-ray photoelectron spectroscopy—detecting of electrons freed from the surface by energetic photons. The electrons and heavier particles have high enough scattering cross sections that they would not survive a trip of any distance through a liquid or gas. Only in vacuum, or at a pressure of less than 10−3 torr, is the particle mean free path long enough that those surface probes can be detected. They then allow the determination of the surface composition, electronic structures and oxidation states of surface atoms, and the structures of adsorbed molecules.
Many major discoveries have come out of the study of surfaces under vacuum. Among them is the phenomenon of surface reconstruction, the rearrangement of surface atoms, due to their reduced atomic coordination, into a structure very different from that of the bulk. Surfaces of metals, semi-conductors, alkali halides, oxides, and even ice were all found to reconstruct.
Adsorbed atoms and molecules were found to occupy sites of stronger bonding on surfaces and cause further restructuring of surface atoms. It was discovered that defects, atomic steps, and kinks in the steps are chemically active, making and breaking chemical bonds with adsorbed molecules with near-unity reaction probability. Surface thermodynamic studies using calorimetry and thermal desorption revealed that the heat of adsorption usually declines with increasing coverage due to interactions among the adsorbed molecules. In bimetallic alloys and other multicomponent systems, one of the components was found to accumulate at the surface in a phenomenon known as surface segregation. Molecular- and atomic-beam scattering studies revealed the nature of energy transfer to the surface in a single collision. And femtosecond photon pulses incident on metal surfaces were found to generate energetic “hot” electrons that carry away much of the photons’ energy before relaxation through lattice vibrations can occur.
Surface science under pressure
Since the 1980s, researchers have worked to develop techniques that can probe the structure, composition, mechanical properties, and dynamics of surfaces at high pressure. 3 To a surface scientist, high pressure means anything greater than the millitorr limit of the vacuum techniques described in the previous section. Two techniques that we examine here in detail are high-pressure scanning tunneling microscopy 4 and sum-frequency-generation (SFG) vibrational spectroscopy. 5 Others include single-crystal catalytic reactors, atomic force microscopy, 6 reflection-adsorption IR spectroscopy, and laser Raman spectroscopy. 7
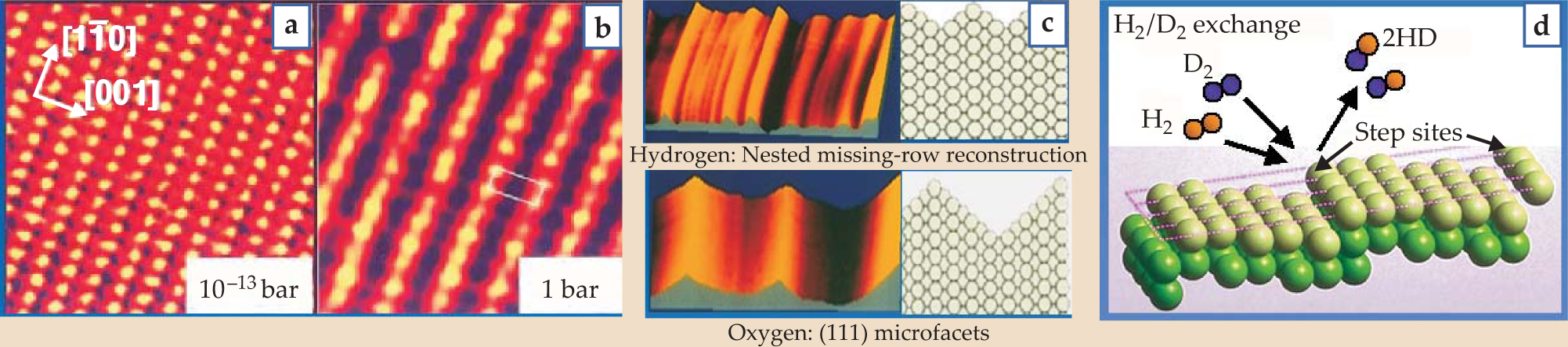
STM images reveal that at high pressures, surfaces form new structures that are not seen under vacuum. As more molecules adsorb onto the surface, the repulsive interaction among them becomes more important and causes the surface to reconstruct in new ways. Flemming Besenbacher and coworkers at the University of Aarhus in Denmark used high-pressure STM to show that at hydrogen pressures greater than 1.5 torr, the copper (110) surface reconstructs into the missing-row structures shown in figures 1(a) and

Figure 1. Scanning tunneling microscopy topographical images showing a copper (110) surface (a) before and (b) during hydrogen exposure. (c) STM topographical images of adsorbate-induced surface reconstructions of platinum (110) surface in 1.7 bar hydrogen and 1.0 bar oxygen. (d) A molecular-beam scattering experiment reveals that the reaction probability of hydrogen/deuterium exchange is three orders of magnitude higher on a stepped surface than on a defect-free Pt(111) crystal face.
(Adapted from ref. 4, Österlund.)
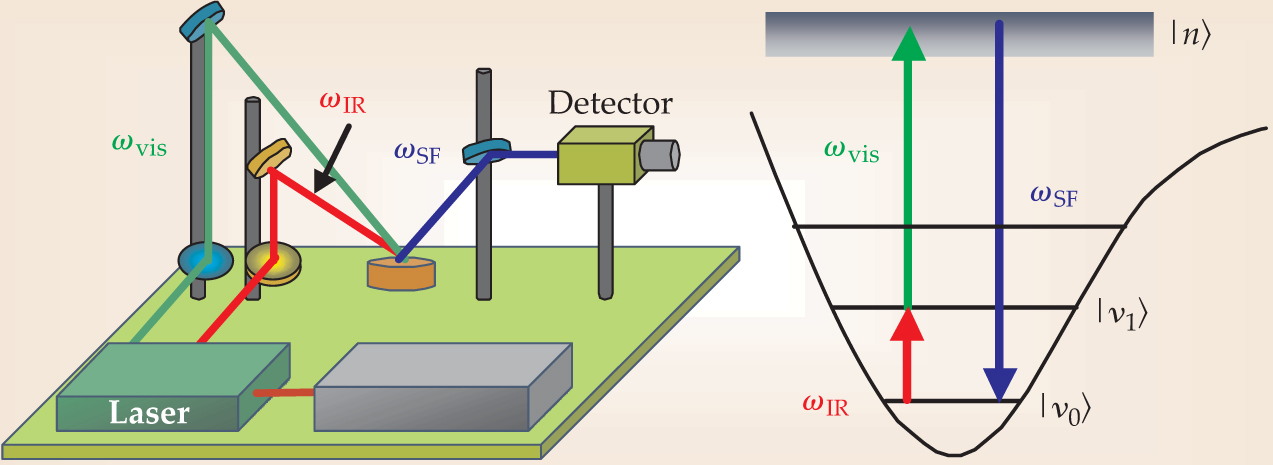
SFG is a nonlinear surface-specific technique developed by Yuen-Ron Shen at the University of California, Berkeley, and used to identify adsorbed molecules by their characteristic vibrational frequencies. 8 The experimental setup is shown in figure 2. Two laser beams are overlapped on the surface, one with a fixed visible frequency ωvis and the other tunable in the IR. When the IR frequency ωIR coincides with a vibrational frequency of an adsorbed molecule, the molecule absorbs an IR photon and a visible photon and is excited to a high-energy virtual state |n〉. The molecule then emits a photon with the sum frequency ωSF. The two-photon transition can only occur in a medium that has no inversion symmetry. The interface satisfies that condition, but the bulk and the isotropic gas or liquid phases do not, so SFG detects only those molecules adsorbed on the surface.

Figure 2. Sum-frequency-generation vibrational spectroscopy identifies adsorbed molecules by their vibrational frequencies. The frequency ωvis of the visible laser beam is kept fixed, and the IR-beam frequency ωIR is varied. When ωIR coincides with a vibrational transition from |v 0〉 to |v 1〉 of an adsorbed molecule, the molecule is excited to a virtual state |n〉 and emits the sum frequency ωSF. Selection rules dictate that the two-photon transition to the virtual state is only allowed in a medium that lacks inversion symmetry, so the technique probes only those molecules adsorbed on the surface.
SFG spectra are virtually pressure independent over a wide range of pressures.
9
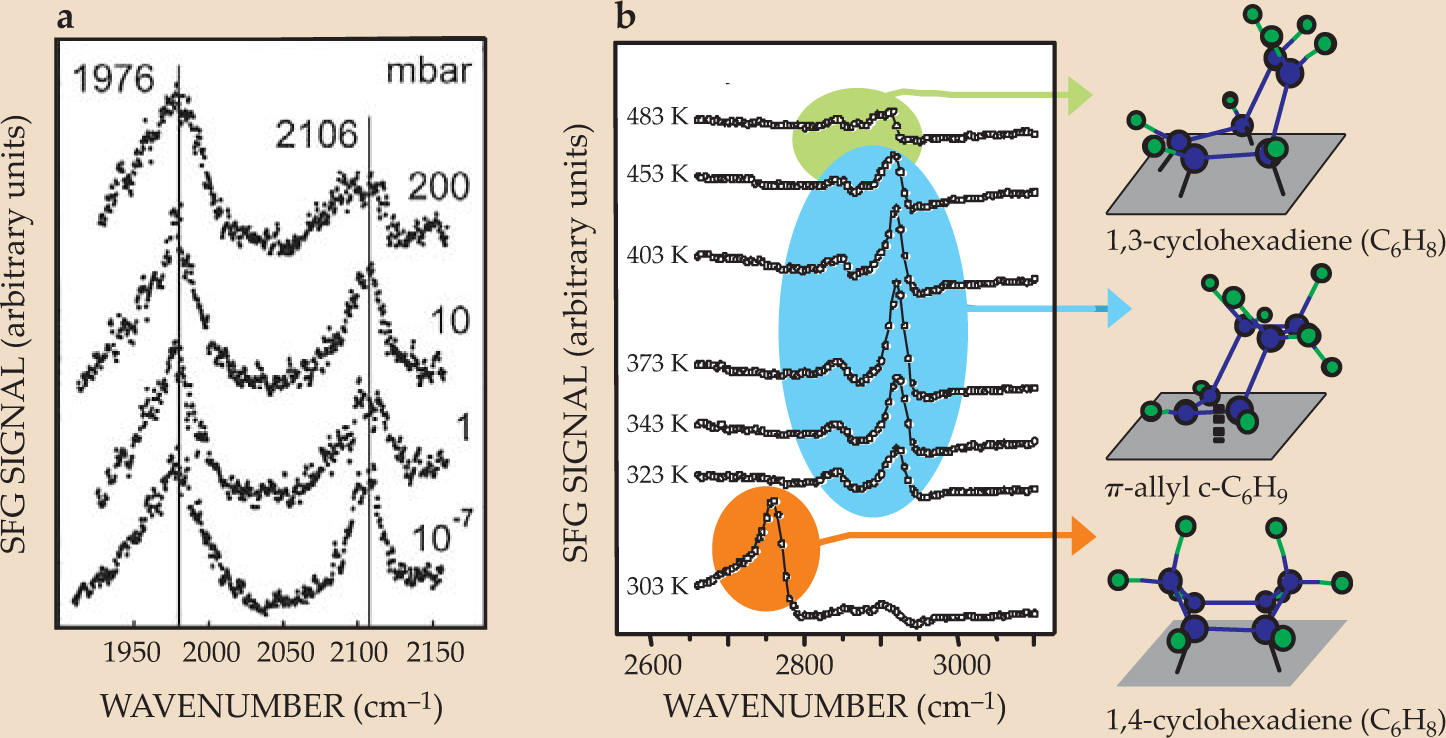
Hans-Joachim Freund and colleagues in the Fritz Haber Institute of the Max Planck Society in Berlin observed that the SFG spectrum of CO on alumina-supported palladium nanoparticles, shown in figure 3(a), did not change over 10 orders of magnitude in pressure.
5
But the real power of SFG lies in its ability to distinguish among different molecular species adsorbed on a surface simultaneously. For example, cyclohexene (C6H10) adsorbed on a surface undergoes two reactions simultaneously: hydrogenation to form cyclohexane (C6H12) and dehydrogenation to form benzene (C6H6). The SFG spectra, shown in figure

Figure 3. Sum-frequency-generation spectra (a) are virtually pressure independent. The SFG spectrum of carbon monoxide on alumina-supported palladium nanoparticles changes only modestly over 10 orders of magnitude in pressure. (b) The SFG spectrum of the platinum (111) surface during cyclohexene hydrogenation reveals three reaction intermediates: 1,3-cyclohexadiene, π-allyl c-C6H9, and 1,4-cyclohexadiene.
((a)Adapted from ref. 5, Dellwig.)
Because surface-based reaction probabilities vary over many orders of magnitude, high-pressure techniques allow the study of chemical reactions that can’t be seen at low pressures. When beams of hydrogen and deuterium are incident on the step sites of a Pt surface such as the one shown in figure
In another dissociation reaction, when methane is incident on a Pt(111) surface, the carbon–hydrogen bonds break, and ultimately a layer of pure carbon is left on the surface. 10 But the dissociation probability at room temperature is less than 10−8. That type of reaction simply cannot be detected at low pressures or in vacuum; a practical study of the reaction requires a minimum of 1 torr of methane colliding continuously with the surface. In general, high reactant pressures are needed to access both the chemically active and less chemically active sites on a surface.
Catalysis and selectivity
Solid surfaces, such as those of transition metals and oxides, carry out chemical reactions over and over again until the reaction is complete or the reaction mixture reaches equilibrium. (See the article by Gerhard Ertl and Freund, Physics Today, January 1999, page 32
A surface acts as a catalyst by rearranging the electrons of the adsorbed molecules, thereby reducing the energy barrier to the reaction. In addition to that electron rearrangement, adsorbate-induced surface reconstruction and the mobility of the adsorbates on the catalyst surface are also essential molecular ingredients of catalytic activity and selectivity. The pressure of the reactant molecules and the surface structure of the catalyst are among the most important variables that affect surface-catalyzed reaction dynamics, with the pressure controlling the reaction probability and the surface structure controlling the nature and concentration of the active sites where the surface reactions occur.
We will briefly describe the surface science of ethylene hydrogenation (H2CCH2 + H2 → H3CCH3) and cyclohexene hydrogenation and dehydrogenation (discussed earlier) on single-crystal Pt(111) and (100) surfaces. Platinum is the grandfather of all catalysts: Its catalytic capability was discovered in the early part of the 19th century. It excels as a catalyst because of its high density of electronic states at the Fermi level, from which it donates and accepts electrons during chemical reactions, and because of its resistance to oxidation and change of valence.
Figure 4(a) shows the SFG spectrum of ethylene during hydrogenation at room temperature under 10 torr of the hydrocarbon and 100 torr of H2. The turnover rate is 10 ethane molecules per Pt site per second. Three species are detectable: ethylidyne (CCH3), in which the free carbon atom forms three bonds to the metal surface; di-σ-bonded ethylene, in which each carbon atom forms a bond with the surface; and π-bonded ethylene, in which a metal atom forms a bond with the electrons already participating in the C–C double bond. Isotope-labeling studies reveal that only the weakly bound π-bonded ethylene goes on to form ethane; the other two species are spectators on the metal surface. The Pt(111) and (100) surfaces carry out the reaction at the same rate, which indicates that the active metal sites are the same in structure and concentration on the two metal surfaces. Ethylene hydrogenation is thus known as a structure-insensitive reaction.

Figure 4. Hydrogenation of ethylene (C2H4) on a platinum surface. (a) Three species are detected in the sum-frequency-generation spectrum from a surface during ethylene hydrogenation. (b, c) The rates of cyclohexene hydrogenation and dehydrogenation differ between the platinum (111) and (100) surfaces. The reactions are surface-structure sensitive. (d) The progress of cyclohexene hydrogenation to cyclohexane and dehydrogenation to benzene is shown on a Pt(111) surface at 350 K. Because the adsorbed molecules are mobile on the surface, the inset scanning tunneling microscopy image shows no surface structure. (e) When carbon monoxide is added to the reaction mixture, the adsorbates lose their mobility, and the reaction does not progress.
(Adapted from ref. 9, Cremer.)
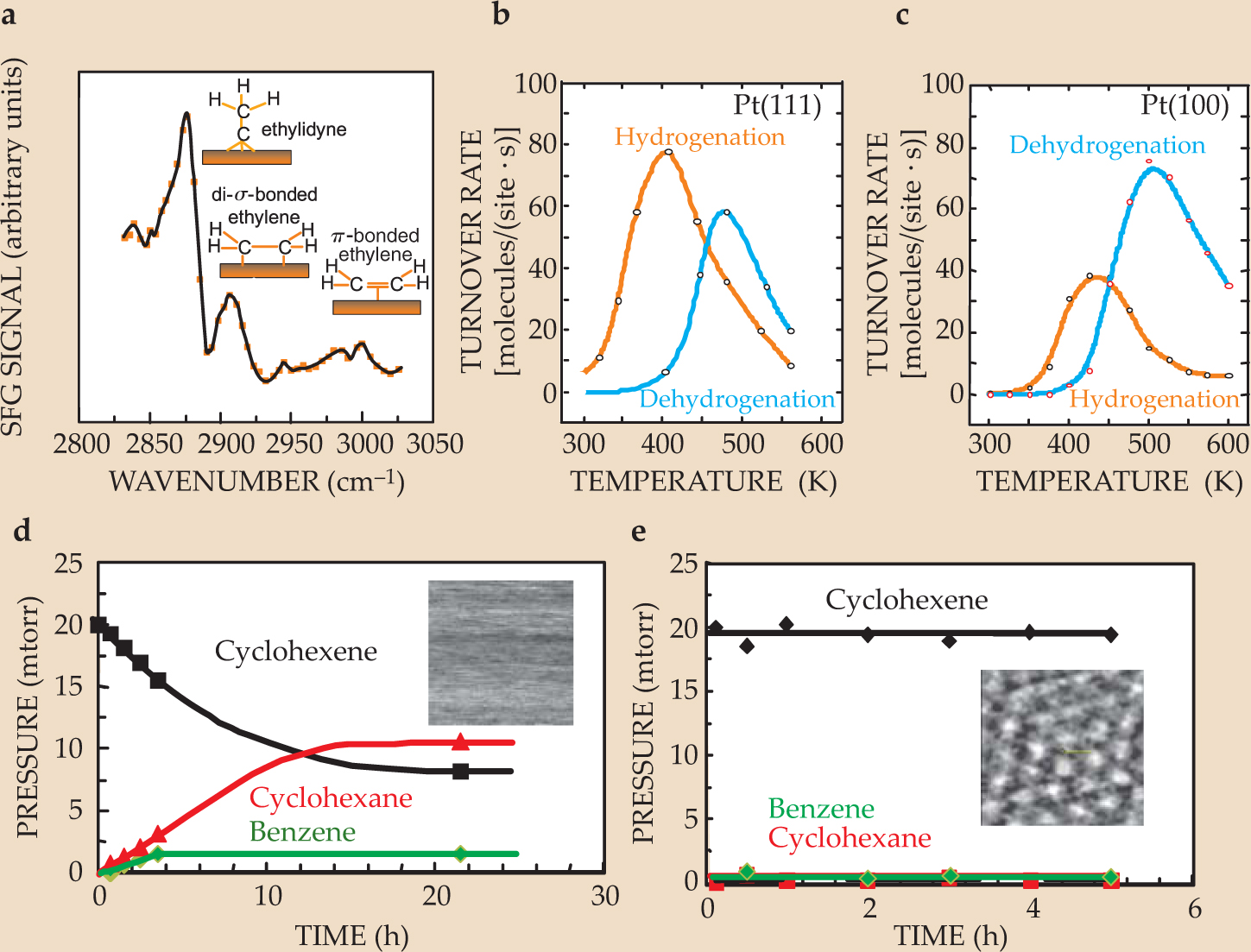
Cyclohexene hydrogenation and dehydrogenation, on the other hand, are surface-structure sensitive, as shown by the reaction rates plotted in figures
The cyclohexene reactions also demonstrate the importance of molecular mobility on the surface. Figure
From single crystals to nanoparticles
Just as the surface of a single crystal can act as a catalyst, so can the surfaces of nanoparticles. A nanoparticle array contains new degrees of freedom—the particle size and shape, and the interface sites between the metal particles and the oxide surface on which they are deposited—that affect the reaction output. Figure 5, for example, shows the dependence of the cyclohexene hydrogenation/dehydrogenation reaction on the size of the platinum-nanoparticle catalyst. That dependence of the reaction products is probably due to the concentration of defects in the metal nanoparticles and the particles’ rapid reconstruction because of their many lower-coordination surface atoms. Control over nanoparticles’ catalytic properties allows for greater reaction selectivity and smaller amounts of waste byproducts.

Figure 5. When cyclohexene hydrogenation and dehydrogenation are catalyzed by platinum nanoparticles, the reaction selectivity depends strongly on the nanoparticle size. The data shown were taken at 480 K, a temperature at which dehydrogenation is the preferred reaction on single-crystal Pt surfaces.
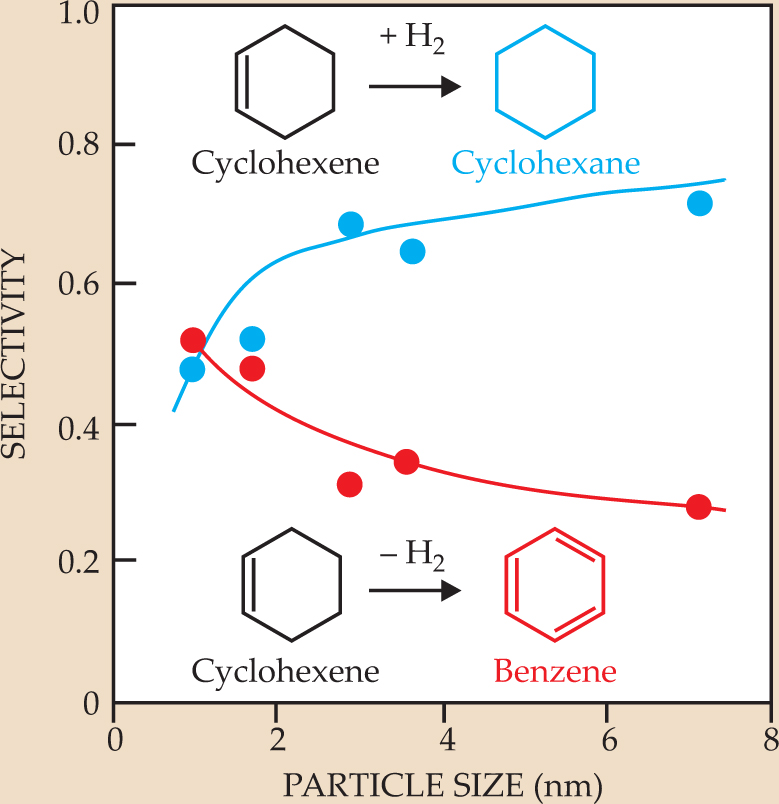
Figure 6 illustrates some of the possibilities for nanostructure catalysts. Nanoparticles are usually prepared by colloid synthesis and capped with a polymer to prevent aggregation in solution. (The polymer cap is porous and allows molecules to enter and exit to undergo catalytic reactions, although some of the metal sites can be blocked.) Two-dimensional arrays of nanoparticles affixed to a substrate can be prepared by the Langmuir–Blodgett technique,
12
described in the

Figure 6. Possibilities for the model catalyst system, from single-crystal metal surfaces, to two- and three-dimensional arrays of colloid-synthesized nanoparticles, to nanostructures fabricated by lithography.
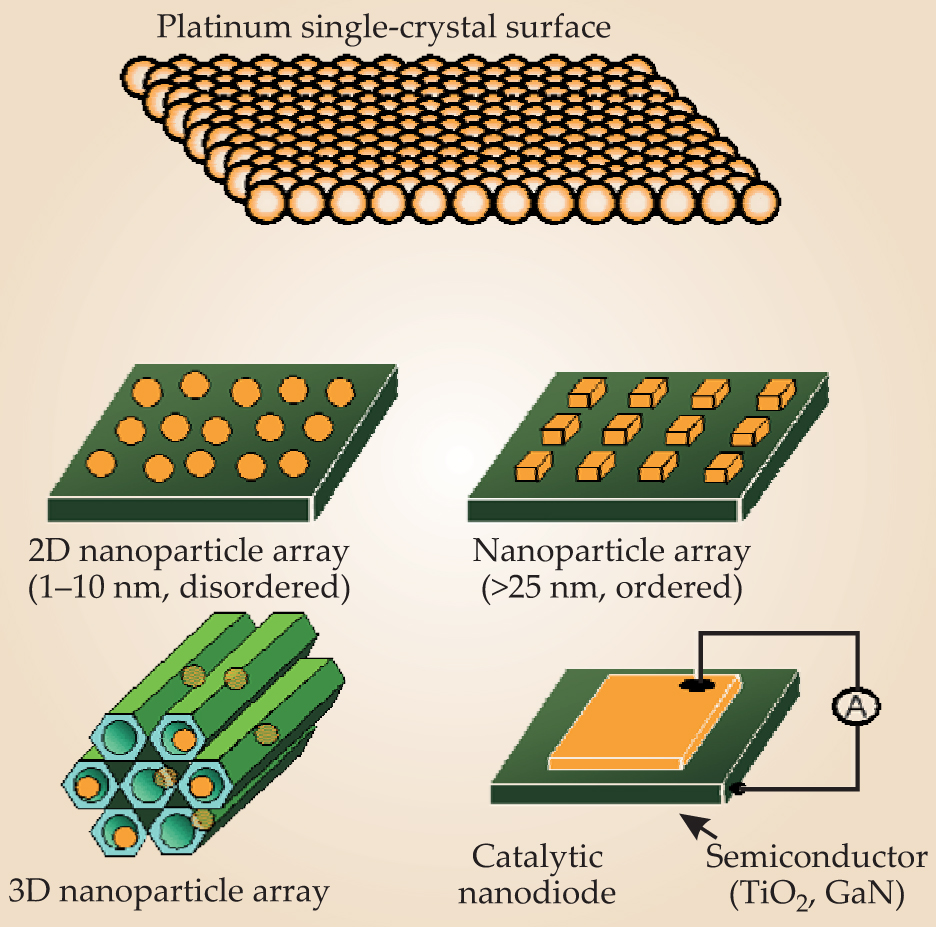
A major advantage of using a diverse range of nanostructures stems from the catalytic activity of the interface between the metal nanostructures and the oxide substrate. Changing the geometry of that interface can allow additional control over activity and selectivity. Chemists have known for decades that when metal nanoparticles are affixed to a catalytically inactive oxide surface, the catalytic turnover rate of the array is more than 10 times that of a metal surface alone. Researchers have exploited that phenomenon empirically by testing different metal–oxide combinations, without fully understanding the mechanism behind the effect.
The Langmuir–Blodgett technique
The Langmuir–Blodgett technique is a means of creating well-defined monolayers on a solid substrate. Its usual application is in the context of molecular monolayers, but here we describe how it is used to form nanoparticle monolayers. The substrate is immersed in the water of the Langmuir trough, and a dilute suspension of nanoparticles, such as platinum cubes, in a volatile solvent is added on top of the water. When the solvent evaporates, a nanoparticle monolayer is left on the water surface. The motorized mobile barrier then compresses the layer of nanoparticles to control the number density, which affects the surface pressure measured by the pressure sensor. As the substrate is withdrawn from the trough, the nanoparticles adhere to it.

Subsequent research has attributed the origin of the oxide–metal interface activity to energetic hot electrons generated at the metal surfaces. When an exothermic chemical reaction is catalyzed by a metal surface, some of the energy released by the reaction is carried away by electrons with kinetic energies of 1–3 eV. In a macroscopic metal structure, the electrons are quickly thermalized, but if the metal’s dimensions are less than 5–10 nm, some of the hot electrons are able to escape the metal and enter the oxide. (Hot electrons generated by nanoparticles in solar cells can be captured to increase the energy conversion efficiency; see the article by George Crabtree and Nathan Lewis, Physics Today, March 2007, page 37
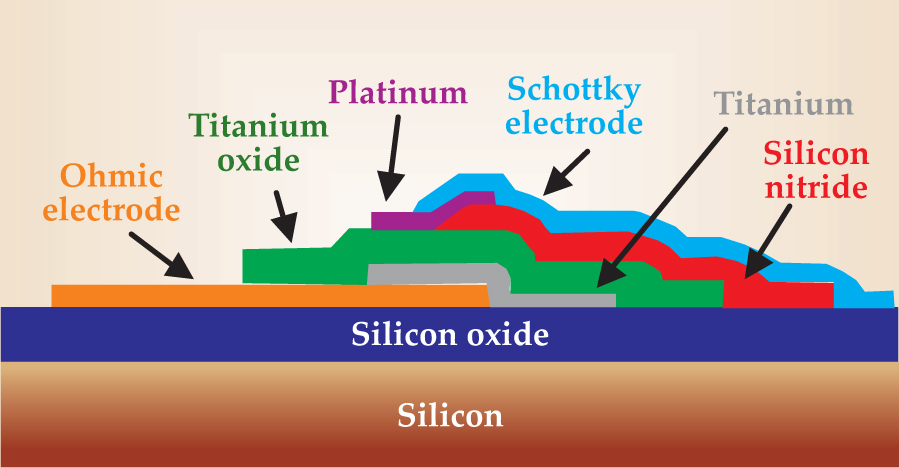
The flow of hot electrons generated by a surface-catalyzed reaction can be observed with the help of a catalytic nanodiode,
13
such as the one shown in figure
Outlook
The developments we have described in this article—the introduction of instruments for studying surfaces at high pressures and the growing understanding of the degrees of freedom offered by nanoparticle systems—opened up opportunities for molecular-level studies in many applications of surface science. Those applications, includingcatalysis, energy conversion, tribology, and polymer surfaces, have given rise to active fields of their own that are growing rapidly as molecular studies reveal new mechanistic information.
Our focus here has been on solid–gas interfaces, but the study of solid–liquid interfaces is an equally active and important research area, due especially to applications in biophysics and electrochemistry. Devices such as contact lenses and artificial heart valves introduce new interfaces into the human body for the sake of increasing the quality and quantity of life. The challenge is making the devices biocompatible to prevent rejection by the body—and biocompatibility appears to correlate with the adsorption of proteins and pep-tides, their surface structure, and their orientation. In the field of surface electrochemistry, researchers are devising ways to monitor the surface structure and the orientation of adsorbed molecules as a function of applied potential. That ability is exploited in the study of corrosion, with the goal of understanding and controlling environmental damage.
The future has never been brighter for surface science. When it is combined with nanosciences to yield an understanding of the atomic and electronic structures of nanoparticles along with their optical, magnetic, and electrical properties, it is likely to become a dominant field, in both pure and applied sciences, for many years to come.

Figure 7. The catalytic nanodiode. The platinum surface catalyzes an exothermic reaction and generates hot electrons. The electrons pass into the titanium oxide layer, thermalize there, and no longer have enough energy to pass back into the Pt film. The result is a one-way chemicurrent proportional to the reaction rate.
This article is based on the Langmuir Award lecture presented by one of us (Somorjai) at the March meeting of the American Physical Society in 2007. We thank the US Department of Energy for support under contract number DE-AC02–05CH11231.
References
1. G. A. Somorjai, R. M. Rioux, Catal. Today 100, 201 (2005); https://doi.org/10.1016/j.cattod.2004.07.059
G. A. Somorjai, M. Yang, Top. Catal. 24, 61 (2003). https://doi.org/10.1023/B:TOCA.0000003077.98309.cf2. J. H. Clark, Green Chem. 1, 1 (1999). https://doi.org/10.1039/a807961g
3. G. A. Somorjai et al., Proc. Natl. Acad. Sci. USA 103, 10577 (2006); https://doi.org/10.1073/pnas.0507691103
G. A. Somorjai et al., Phys. Chem. Chem. Phys. 9, 3500 (2007). https://doi.org/10.1039/b618805b4. L. Österlund et al., Phys. Rev. Lett. 86, 460 (2001);
B. L. M. Hendriksen, J. W. M. Frenken, Phys. Rev. Lett. 89, 046101 (2002); https://doi.org/10.1103/PhysRevLett.89.046101
J. A. Jensen et al., Phys. Rev. Lett. 80, 1228 (1998). https://doi.org/10.1103/PhysRevLett.80.12285. T. Dellwig et al., Phys. Rev. Lett. 85, 776 (2000); https://doi.org/10.1103/PhysRevLett.85.776
X. Su et al., J. Am. Chem. Soc. 119, 3994 (1997). https://doi.org/10.1021/ja96387236. O. Mermut et al., J. Am. Chem. Soc. 128, 3598 (2006); https://doi.org/10.1021/ja056031h
J. Y. Park et al., Science 309, 1354 (2005). https://doi.org/10.1126/science.11132397. F. M. Mirabella, ed., Internal Reflection Spectroscopy: Theory and Applications, Marcel Dekker, New York (1993);
M. Moskovits, Rev. Mod. Phys. 57, 783 (1985). https://doi.org/10.1103/RevModPhys.57.7838. Y. -R. Shen, Nature 337, 519 (1989); https://doi.org/10.1038/337519a0
Annu. Rev. Phys. Chem. 40, 327 (1989). https://doi.org/10.1146/annurev.pc.40.100189.0015519. K. McCrea et al., Surf. Sci. 494, 238 (2001); https://doi.org/10.1016/S0039-6028(01)01469-8
P. S. Cremer et al., J. Am. Chem. Soc. 118, 2942 (1996); https://doi.org/10.1021/ja952800t
M. Yang, K. C. Chou, G. A. Somorjai, J. Phys. Chem. B 107, 5267 (2003). https://doi.org/10.1021/jp034355r10. S. L. Bernasek, G. A. Somorjai, J. Chem. Phys. 62, 3149 (1975); https://doi.org/10.1063/1.430862
A. L. Marsh, K. A. Becraft, G. A. Somorjai, J. Phys. Chem. B 109, 13619 (2005). https://doi.org/10.1021/jp051718+11. K. R. McCrea, G. A. Somorjai, J. Mol. Catal. A: Chem. 163, 43 (2000); https://doi.org/10.1016/S1381-1169(00)00398-8
M. Montano, M. Salmeron, G. A. Somorjai, Surf. Sci. 600, 1809 (2006). https://doi.org/10.1016/j.susc.2006.02.02612. J. A. Zasadzinski et al., Science 263, 1726 (1994); https://doi.org/10.1126/science.8134836
H. Song et al., J. Phys. Chem. B 109, 188 (2005). https://doi.org/10.1021/jp046477513. J. Y. Park, G. A. Somorjai, Chem. Phys. Chem. 7, 1409 (2006); https://doi.org/10.1002/cphc.200600056
X. Ji et al., Nano Lett. 5, 753 (2005); https://doi.org/10.1021/nl050241a
H. Nienhaus et al., Phys. Rev. Lett. 82, 446 (1999). https://doi.org/10.1103/PhysRevLett.82.446
More about the authors
Gabor Somorjai is a professor of chemistry and a University Professor at the University of California, Berkeley, and a faculty senior scientist in the materials sciences division of Lawrence Berkeley National Laboratory. Jeong Young Park is a staff scientist in the department of chemistry at the University of California, Berkeley, and Lawrence Berkeley National Laboratory.
Gabor A. Somorjai, 1 University of California, Berkeley, US .
Jeong Y. Park, 2 University of California, Berkeley, and Lawrence Berkeley National Laboratory, US .




