Fractional Kinetics
DOI: 10.1063/1.1535007
Until about 10 years ago, expressions involving fractional derivatives and integrals were pretty much restricted to the realm of mathematics. But over the past decade, many physicists have discovered that a number of systems—particularly those exhibiting anomalously slow diffusion, or subdiffusion—are usefully described by fractional calculus. Those systems include charge transport in amorphous semiconductors, the spread of contaminants in underground water, relaxation in polymer systems, and tracer dynamics in polymer networks and in arrays of convection rolls.
Fractional diffusion equations generalize Fick’s second law and the Fokker–Planck equation by taking into account memory effects such as the stretching of polymers under external fields and the occupation of deep traps by charge carriers in amorphous semiconductors. Such generalized diffusion equations allow physicists to describe complex systems with anomalous behavior in much the same way as simpler systems.
Fractional calculus
Physicists are all familiar with the high-school calculus that introduces students to derivatives of integer order
In fact, the calculus of fractional integrals and derivatives is almost as old as calculus itself. As early as 1695, Gottfried von Leibnitz, in a reply to Guillaume de l’Hôpital, wrote, “Thus it follows that
One way to formally introduce fractional derivatives proceeds from the repeated differentiation of an integral power:
For an arbitrary power μ, repeated differentiation gives
The extension defined by equation
A second, more elegant and general way to introduce fractional derivatives uses the fact that the nth derivative is an operation inverse to an n-fold repeated integration. Basic is the integral identity
Clearly, the equality is satisfied at x = a, and it is not difficult to see iteratively that the derivatives of both sides of the equality are equal. A generalization of the expression allows one to define a fractional integral of arbitrary order α via
A fractional derivative of an arbitrary order is defined through fractional integration and successive ordinary differentiation. For additional elaboration, see
Examples of fractional calculus with
| Semi-integral | Function | Semi-derivative |
|---|---|---|
|
|
|
|
|
|
C, any constant |
|
|
|
|
0 |
|
|
|
|
|
|
x |
|
|
|
|
|
|
|
exp(x) |
|
|
|
ln x |
|
Examples of fractional calculus with
C, any constant |
||
0 |
||
x |
||
exp(x) |
||
ln x |
In this article, we are generally concerned with fractional time derivatives, and we set a = 0, in effect choosing t = 0 as the beginning of the system’s time evolution. In particular, we note that the operator
Definitions and examples
The fractional integration operator
The number of additional differentiations n is equal to [α] + 1, where [α] is the whole part of α. From the above definition it follows that
That is, the derivative of a constant vanishes only if the order of the derivative is integer, in which case Γ(1 – α) diverges. Another interesting result holds for the derivative of the exponential function:
The practical use of fractional calculus is underlined by the fact that, under Laplace transform, the operator
The result for the differentiation of an exponential may seem disappointing. But if one chooses the lower limit of integration to be a = − ∞ in equation
We see that there are several ways to interpret “
From Fick to fractional diffusion
The foundations of kinetics were established more than 150 years after the prophecy of Leibnitz, without the use of fractional calculus. In 1855, the young Adolf Fick, a pathologist at the University of Zürich, wrote a work entitled “Über Diffusion” (“On Diffusion”). The work was published in Poggendorf’s Annalen der Physik, the Physical Review Letters of that time. Fick started by observing that “diffusion in water confined by membranes is not only one of the basic factors of organic life, but it is also an extremely interesting physical process and, as such, should attract much more attention from physicists than it has so far.” Diffusion processes such as those considered by Fick, and processes that are described by fractional calculus, continue to fascinate physicists and others.
Fick was an experimental physiologist, but his work on diffusion was theoretical, and his approach would today be called a phenomenological linear-response theory applied to diffusion. In brief, the result of diffusion is known to be the equilibration of concentrations. Thus, particle current has to flow against the concentration gradient. In analogy with Ohm’s law for electric current, or with Fourier’s law for heat flow, Fick assumed that the current j is proportional to the concentration gradient, so that
Combining Fick’s first law with the continuity equation gives Fick’s second law, also known as the diffusion equation:
Fick’s phenomenology missed the probabilistic point of view central to statistical mechanics. It was Albert Einstein who, 50 years after “On Diffusion,” first derived the diffusion equation from the postulates of molecular theory, in which particles move independently under the influence of thermal agitation. In his picture, the concentration of particles c(r, t) at some point r is proportional to the probability P(r, t) of finding a particle there. Thus, the diffusion equation holds when probabilities are substituted for concentrations.
If, for example, a particle is initially placed at the origin of coordinates in d-dimensional space, then its evolution, according to equation
The mean squared displacement of the particle is thus
Note that the scaling form 〈r 2〉 ∝ t follows directly from the structure of the diffusion equation. That equation is second order in the spatial coordinates and first order in time: Changing the spatial scale by a factor of 3 corresponds to changing the time scale by a factor of 9.
In a variety of physical systems, however, the simple scaling pertinent to Fickian diffusion is violated.
3,4
The mean squared displacement grows as
Continuous-time random walks
Random walks and diffusion serve as an interface between kinetics on one hand and derivatives and integrals of fractional order on the other. The simplest model leading to normal diffusion is the random walk. Related models were introduced in Lord Rayleigh’s studies of isoperiodic vibrations (1880) and in Louis Bachelier’s analysis of stock-market fluctuations (1900). If a random walker strolling in one dimension moves a step of length a in either direction precisely when each unit time elapses, then the displacement after a large number of steps (that is, after a long time) will be distributed according to the Gaussian, equation
In continuous-time random walks (CTRWs)—introduced in physics by Elliot Montroll and George Weiss—the condition that the steps occur at fixed times is relaxed. 5 Rather, the time intervals between consecutive steps are governed by a waiting-time distribution Ψ(t). In describing transport, the Ψ(t) distributions may stem from possible obstacles and traps that delay the particle’s jumps and thus introduce memory effects into the motion. If the mean waiting time between consecutive steps is finite, τ = ∫ t Ψ(t)dt < ∞, the CTRW is described by Fick’s diffusion equation, with the diffusion coefficient κ equal to α 2/2τ.
The situation changes drastically if the mean waiting time diverges, as is the case for power-law waiting-time distributions of the form

Figure 1. Continuous-time random walks (CTRWs) do not cover ground as quickly as simple random walks. The black lines indicate a specific realization of a simple random walk (left) and a CTRW (right). Note that CTRW steps occur very irregularly; most of the time the walker doesn’t move at all. As a consequence, the mean square displacement in CTRWs grows considerably slower than in simple random walks. The blue and the red curves indicate the typical behavior of the displacement:
Transport phenomena in systems exhibiting subdiffusion have α < 1, whereas systems that exhibit superdiffusion have α > 1.
Fractional modifications of the commonly used diffusion and Fokker–Planck equations generate the scaling behavior seen in subdiffusive systems. Consider, for example, the fractional equation first introduced by Venkataraman Balakrishnan and by W. R. Schneider and W. Wyss:
6
Equation
Fractional Fokker–Planck equation
The standard diffusion equation accounts for a particle’s motion due to uncorrelated molecular impacts. In many cases, an external deterministic force is imposed on a system in addition to such random impacts. The Fokker–Planck equation considers both contributions.
9
It can be derived by combining Fick’s first law—expressed in terms of probability current and taking into account the external force—with the continuity equation. The probability current is
In the absence of an external force, f = 0, the Fokker–Planck equation reduces to the standard diffusion equation.
In parallel to the diffusion case, one can generalize the Fokker–Planck equation to
In equilibrium, the current j must vanish. After expressing the force in terms of a potential function U(r), one may write the equilibrium probability distribution that satisfies equation
For example, consider a constant external force acting in the x direction. The force leads to a mean drift,
The expression above is the fluctuation-dissipation theorem, which holds for subdiffusion in the fractional Fokker–Planck framework.
Ornstein–Uhlenbeck processes in one dimension provide a second example. Such processes involve diffusion in the harmonic potential
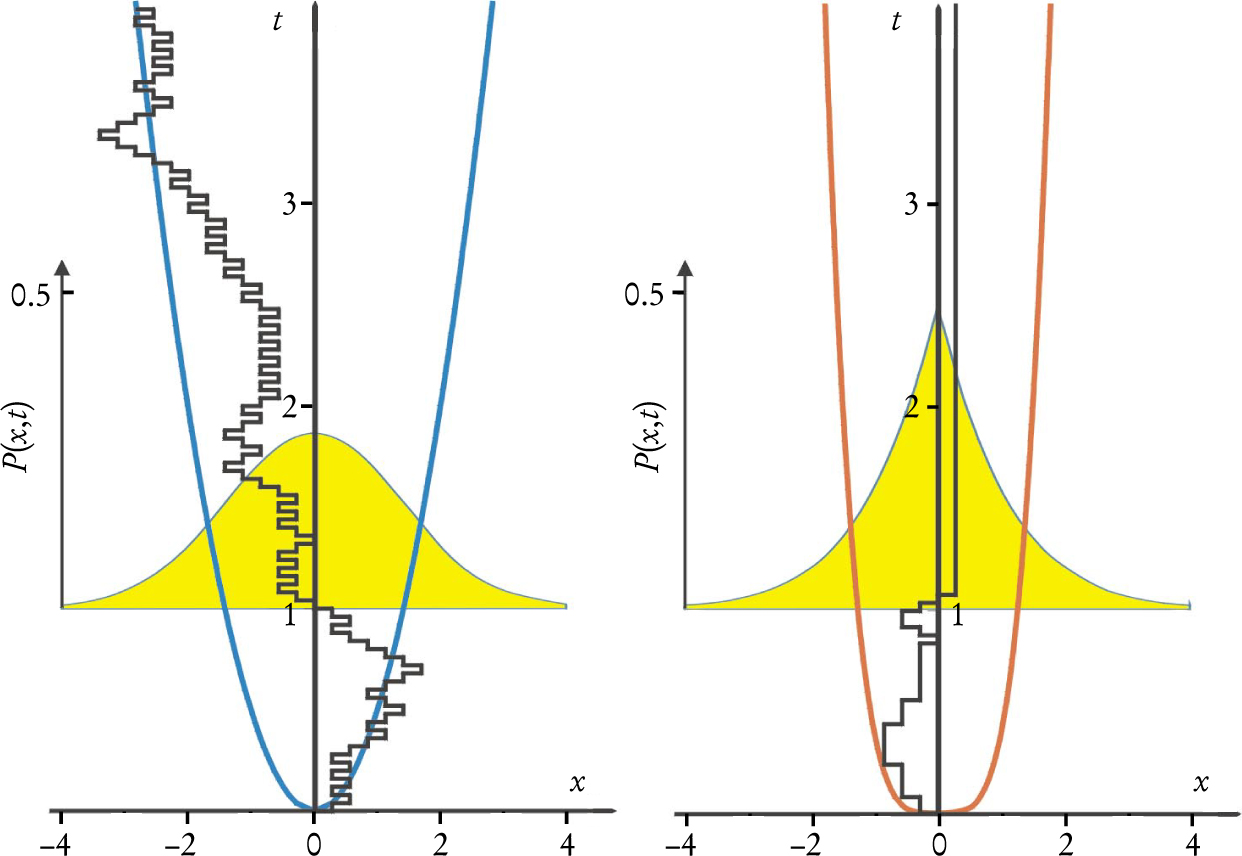
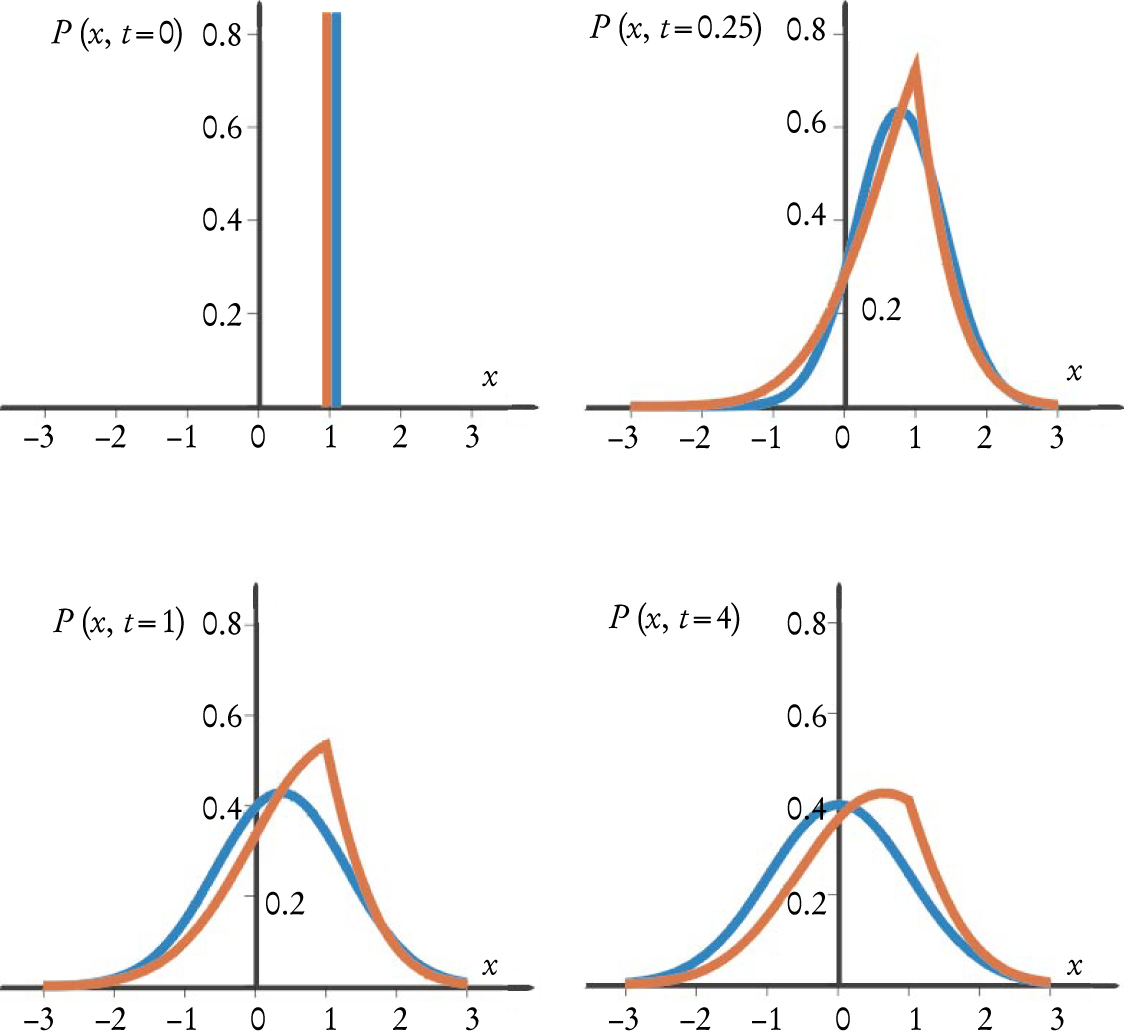
Figure 2 shows snapshots of the time-dependent probability distribution for particles originally at a well-defined location, according to both the fractional and conventional Fokker–Planck equations with harmonic potentials. In both cases, the asymptotic forms of the distribution are Gaussian, because the Fokker–Planck equations describe the relaxation of thermodynamic systems to equilibrium. In the fractional case, though, the distribution at finite times has a cusp characteristic of broad CTRW distributions.

Figure 2. Probability distributions evolve as particles governed by Fokker–Planck equations with harmonic potentials relax toward Boltzmann equilibrium. The graphs at left (with position x and time t in arbitrary units) show the evolution of the probability distribution for a particle collection initially prepared at x = 1. For both the regular (blue) and α =
In thermodynamic applications, one is mostly interested in mean values—for example, the mean particle position
It follows that 〈x(t)〉 decays exponentially toward equilibrium: 〈x(t)〉 = 〈x(0)〉 exp(–t/τ).
In the case of diffusion governed by the fractional Fokker–Planck equation, the mean displacement obeys

Figure 4. A particle diffusing in a harmonic potential exemplifies thermodynamic relaxation. For regular diffusion, the mean value of the particle’s position decays exponentially with time (blue). In the fractional, subdiffusive case, the relaxation is described by a Mittag–Leffler function. The particular Mittag–Leffler function leading to the relaxation illustrated in red has as its fractional parameter α =


Figure 3. The Mittag–Leffler function describes the relaxation toward equilibrium of particles governed by the fractional Fokker–Planck equation. For t close to zero, the function behaves like a stretched exponential,

From Oliver Heaviside to Stress Moduli of polymers
Fractional derivatives often emerge in the description of self-similar, hierarchically organized systems. Consider a discrete model for a transatlantic telegraph cable, as proposed by Lord Kelvin. The model consists of identical resistances R and identical capacitors C, illustrated in the figure at right. The response of the cable to a voltage V(t) applied at, say, its right end may be related to the impedance Z of the system. The relation between impedance, current I, and the voltage is most simply expressed in terms of the Fourier-transformed functions:

Calculating Z(ω) is a standard problem in the theory of electrical circuits. For the transatlantic cable model, the solution is
As can be confirmed by referring to equation
The mechanical equivalent of Kelvin’s model, a chain consisting of springs and beads immersed in a viscous fluid, is the standard Rouse model in polymer dynamics. That model, illustrated below the transatlantic cable, accounts for the fluctuating forces due to solvent molecules, and for viscous friction.
If a force
More complicated behavior arises when several forces act simultaneously—for example, when the monomers of a network are randomly charged and exposed to an external voltage. In such cases, one still observes scaling, but with values of α that depend on the distribution of the charges on the polymer. Many complicated systems can be modeled using fractional differential equations whose parameters depend on α. For example, the

Applications
The regular Ornstein–Uhlenbeck process is a good model for the behavior of a diffusing particle trapped in optical tweezers: The tweezers create an approximately harmonic well. The corresponding relaxation patterns display exponential decays, as shown by Roy Bar-Ziv and colleagues from the Weizmann Institute of Science in Rehovot, Israel. Particles moving according to the fractional Ornstein–Uhlenbeck equation (equation
One can expect applications of the fractional Fokker–Planck equation in chemical and biological systems. As early as 1916, Marian Smoluchowski showed how the rates of chemical reactions can be determined by imposing boundary conditions on diffusion equations. Because the fractional Fokker–Planck equation handles boundary value problems in the same way as its regular counterpart does, it is a valuable tool for describing reactions in complex systems, as recently reported by groups led by Katja Lindenberg at the University of California, San Diego, and Bob Silbey at the Massachusetts Institute of Technology. 10
Many environmental studies are complicated by a poor understanding of the diffusion of contaminants in complex geological formations. Experiments point to anomalous diffusion. They suggest the need for fractional diffusion–advection equations, 11 which may help scientists to understand and predict the long-term impact of pollution on ecosystems. 12
Detailed studies of the fractional Kramers problem represent another possible arena for the application of fractional kinetics. The problem concerns the escape of a particle over a potential barrier. First steps toward its solution have been achieved by Ralf Metzler and Yossi Klafter, both working at Tel Aviv University.
Fractional calculus can be applied to many areas of physics, other than fractional kinetics. In fact, fractional calculus was introduced in a heuristic manner long ago in rheology (for example, by Andrew Gemant in 1936) in an effort to describe linear viscoelasticity through the extension of the material-dependent constitutive equations used in the field.
13
What about superdiffusion? Fractional generalizations of the diffusion and Fokker–Planck equations have been introduced for superdiffusion as well. Those equations, which apply spatial fractional derivatives rather than temporal ones, are intimately related to Lévy processes in space. George Zaslavsky has advocated using such equations to describe chaotic diffusion in Hamiltonian systems, and other applications have been discussed by Hans Fogedby and by Bruce West and Paolo Grigolini. 1,14 We hasten to note, however, that superdiffusion is far from being completely understood.
The classical diffusion laws derived by Fick dominated physicists’ views on diffusion and transport for more than a century. But recent observations have clearly demonstrated that Fick’s laws have exceptions. Those exceptions, which have been termed strange kinetics,
3
require a completely fresh view of kinetic processes, based on random-walk approaches and on unconventional distribution functions (see the article by Joseph Klafter, Michael F. Shlesinger, and Gert Zumofen, Physics Today, February 1996, page 33
We thank our colleagues Eli Barkai, Christian Friedrich, Ralf Metzler and Helmut Schiessel for fruitful discussions.
References
1. R. Hilfer, ed., Applications of Fractional Calculus in Physics, World Scientific, River Edge, N. J. (2000).
2. K. B. Oldham, J. Spanier, The Fractional Calculus, Academic Press, San Diego, Calif. (1974);
K. S. Miller, B. Ross, An Introduction to the Fractional Calculus and Fractional Differential Equations, Wiley, New York (1993).3. M. F. Shlesinger, G. M. Zaslavsky, J. Klafter, Nature 363, 31 (1993).https://doi.org/10.1038/363031a0
4. J.-P. Bouchaud, A. Georges, Phys. Rep. 195, 127 (1990).https://doi.org/10.1016/0370-1573(90)90099-N
5. E. W. Montroll, G. H. Weiss, J. Math. Phys. 10, 753 (1969);https://doi.org/10.1063/1.1664902
E. W. Montroll, M. F. Shlesinger, in Nonequilibrium Phenomena II: From Stochastics to Hydrodynamics, J. L. Lebowitz, E. W. Montroll, eds., North Holland, New York (1984);
E. W. Montroll, B. J. West, in Fluctuation Phenomena, E. W. Montroll, J. L. Lebowitz, eds., North Holland, New York (1987).6. V. Balakrishnan, Physica A 132, 569 (1985);https://doi.org/10.1016/0378-4371(85)90028-7
W. R. Schneider, W. Wyss, J. Math. Phys. 30, 134 (1989).https://doi.org/10.1063/1.5285787. R. Metzler, J. Klafter, Phys. Rep. 339, 1 (2000).https://doi.org/10.1016/S0370-1573(00)00070-3
8. E. Barkai, Phys. Rev. E 63, 046118 (2001);https://doi.org/10.1103/PhysRevE.63.046118
I. M. Sokolov, Phys. Rev. E 63, 056111 (2001).https://doi.org/10.1103/PhysRevE.63.0561119. H. Risken, The Fokker–Planck Equation: Methods of Solution and Applications, Springer-Verlag, New York (1996).
10. S. B. Yuste, K. Lindenberg, Phys. Rev. Lett. 87, 118301 (2001);https://doi.org/10.1103/PhysRevLett.87.118301
J. Sung, E. Barkai, R. J. Silbey, S. Lee, J. Chem. Phys. 116, 2338 (2002).https://doi.org/10.1063/1.144829411. J. W. Kirchner, X. Feng, C. Neal, Nature 403, 524 (2000).https://doi.org/10.1038/35000537
12. R. Metzler, J. Klafter, I. M. Sokolov, Phys. Rev. E 58, 1621 (1998).https://doi.org/10.1103/PhysRevE.58.1621
13. C. Friedrich, H. Schiessel, A. Blumen, in Advances in the Flow and Rheology of Non-Newtonian Fluids, D. A. Siginer, D. De Kee, R. P. Chhabra, eds., Elsevier, New York (1999), p. 429.https://doi.org/10.1016/S0169-3107(99)80038-0
14. G. M. Zaslavsky, Chaos 4, 25 (1994);https://doi.org/10.1063/1.166054
H. C. Fogedby, Phys. Rev. Lett. 73, 2517 (1994).https://doi.org/10.1103/PhysRevLett.73.2517
More about the authors
Igor Sokolov is a professor of physics at Humboldt University in Berlin, Germany
Igor M. Sokolov, 1 Humboldt University, Berlin, Germany .
Joseph Klafter, 2 Tel Aviv University, Israel .
Alexander Blumen, 3 University of Freiburg, Germany .




