Colloids out of equilibrium
DOI: 10.1063/PT.3.4901
Spontaneous ordering and formation of superstructures in nature have long inspired physicists, chemists, and materials scientists. The intricate ordering of atomic and molecular building blocks allows for the realization of a seemingly infinite number of different materials, each with its own properties and functions. From hard rocks and gemstones to squishy cells and tissues, all result from a hierarchical organization of their elementary constituents.

Confocal microscopy image of banana-shaped colloidal particles with colors added to represent the different orientations of the bananas’ short axis. Because the particles vary significantly in curvature and length, they form a globally disordered phase with multiple domains. (Courtesy of Carla Fernández Rico.)

That library of structures can be expanded even further by considering not only atoms and molecules but also colloids as building blocks. Colloids—or more correctly speaking colloidal particles—are relatively small entities, with characteristic dimensions of 10–1000 nm, that are dispersed in a continuous medium. 1 Examples of colloidal systems from our daily lives include milk (liquid fat droplets in water), paint (solid particles in water), and smoke (solid particles in air). Despite being significantly larger than atoms and typical molecules, colloidal particles are also subject to Brownian motion that originates from thermal fluctuations of the surrounding medium. 2 They can be considered, therefore, as atoms’ and molecules’ larger analogues.
Facilitated by their continuous and autonomous movements, colloids can self-assemble like atoms and molecules into larger superstructures. The presence of mesoscopic length scales endows such supracolloidal materials with unique and tunable optical and mechanical properties. For example, adjusting the distribution of colloidal filler particles is a powerful way to increase the strength of otherwise weak organic materials such as plastics.
3
Natural supra-colloidal materials include opals and the wings of certain butterflies, in which the periodic arrangement of submicron features generates bright colors (see figure
Figure 1.

Microstructural elements of colloidal length scales give opals (top left) and certain butterflies (top right) their vivid colors. (Adapted from H. Inan et al., Chem. Soc. Rev. 46, 366, 2017, doi:10.1039/C6CS00206D
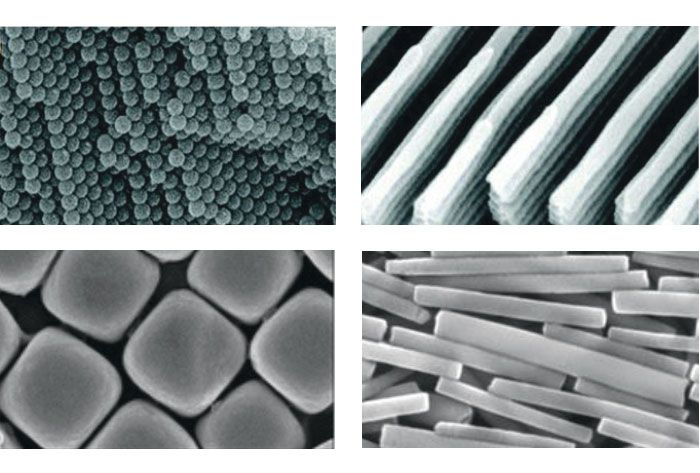
Inspired by the analogy between colloids and atoms or molecules and by the prospect of fabricating materials with new properties, condensed-matter physicists and chemists have started focusing on the controlled self-assembly of colloidal particles. Procedures for synthesizing highly uniform and well-defined building blocks have opened a path to myriad new superstructures. They range from close-packed crystals to low-density ordered arrays and finite-sized clusters
4
(see figure
From opals to cells
Although the progress made in the field of colloidal self-assembly is impressive, the functionalities of the resulting materials remain rather limited. That state of affairs becomes more apparent when compared with an average living cell that is able to dynamically reconfigure, adapt, amplify signals, self-replicate, and self-heal (see figure
Figure 2.

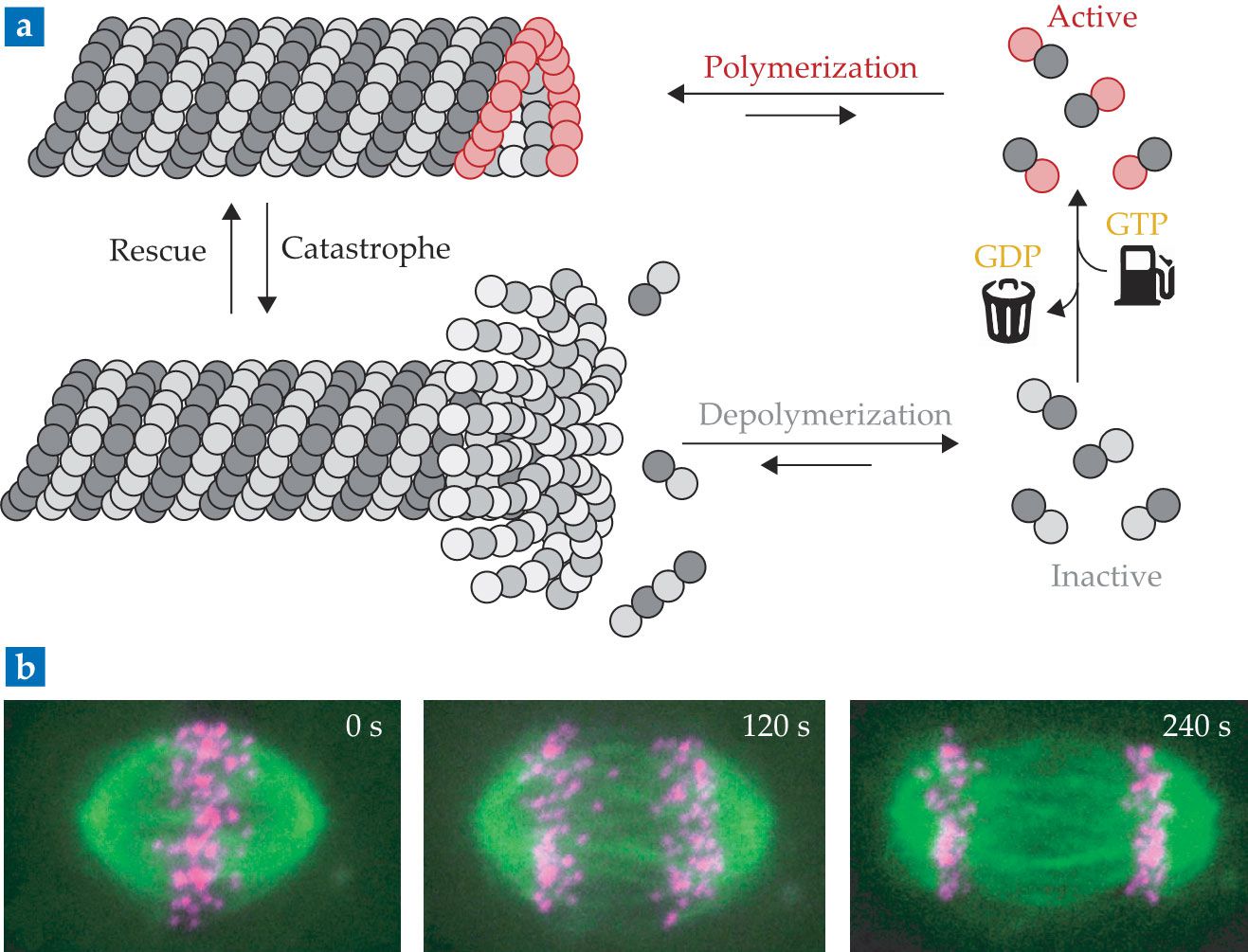
Polymerization of tubulin to form microtubules is fueled by guanosine triphosphate (GTP) (a). Only when one of the two forms of tubulin is in the fuel-activated state (red circles) can polymerization of the elementary dimers into fibers occur. After incorporation, the active building blocks decay to their inactive state, in which GTP converts to the waste product guanosine diphosphate (GDP). The conversion destabilizes the assembly unless the growing end is capped with active dimers. (Adapted from Nordicbiosite.com.) (b) Shown here are high-resolution fluorescence microscopy images of microtubules in action during cell mitosis. (Adapted from K. Vukušić et al., Dev. Cell 43, 11, 2017, doi:10.1016/j.devcel.2017.09.010
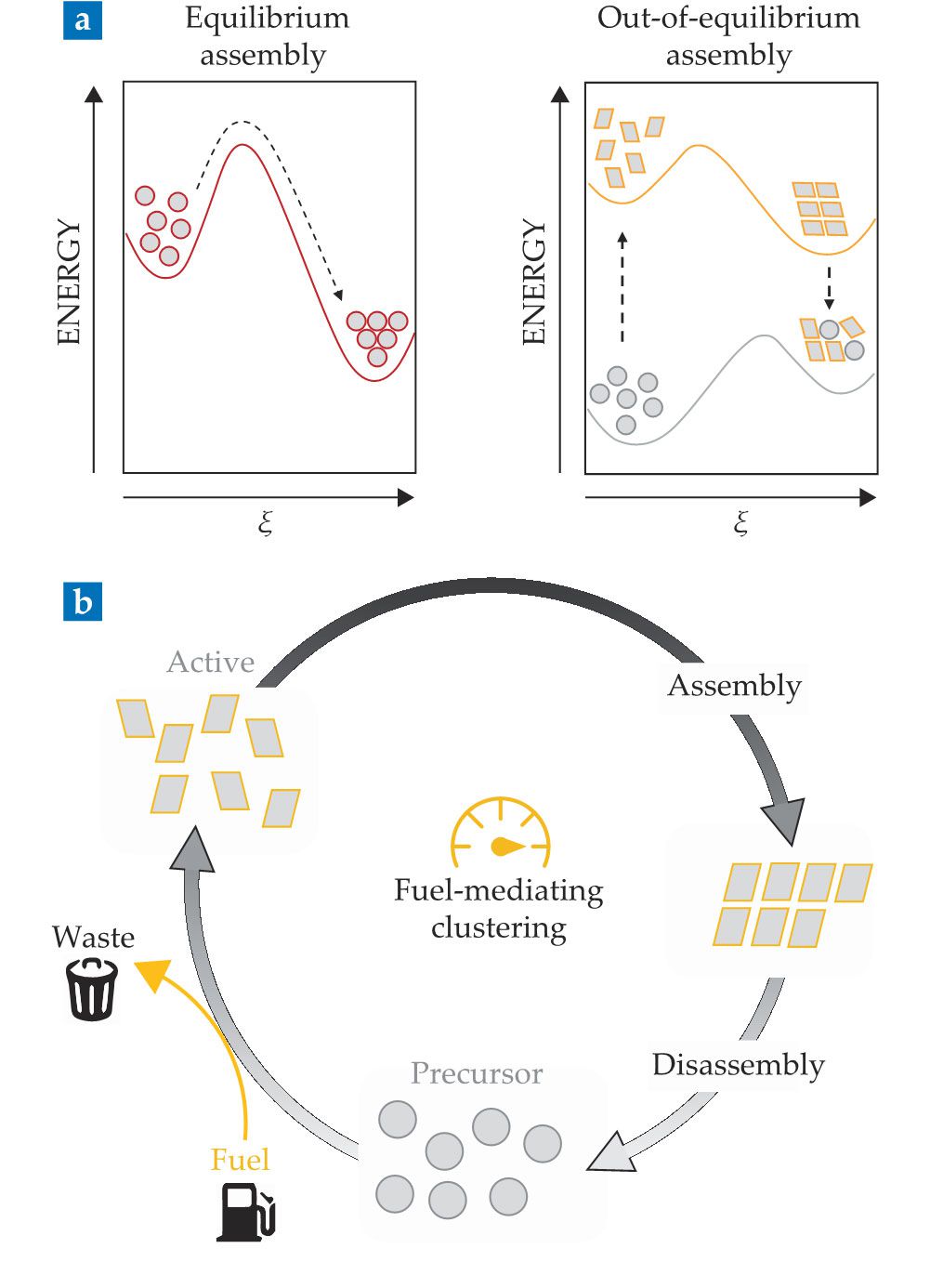
That systems of self-assembling colloids are typically designed based on thermodynamic principles implies that the targeted superstructure exists in or near the global minimum of the free-energy landscape (see figure
Figure 3.

Self-assembly can occur (a) in equilibrium or out of equilibrium. The two plots represent an energy landscape as a function of the reaction coordinate ξ. In equilibrium self-assembly, the assembled structure occupies a global minimum of the energy landscape and is therefore thermodynamically favored. In dissipative assembly (b), building blocks in the precursor state (gray circles) are promoted into their activated state (yellow rectangles) after consuming fuel. Once activated, their assembly commences. The assembled structure does not exist in a global minimum of the energy landscape and is therefore metastable. Once the fuel is depleted, the activated state of the building blocks can no longer be sustained. The structure spontaneously disintegrates and returns to its equilibrium state of well-dispersed precursors. (Adapted from refs.
Because the final structures exist in a deep free-energy minimum, they are stable. The stability is manifested by the absence of major structural rearrangements in response to small external fluctuations. Hence, from the perspective of structural dynamics, typical colloidal self-assembled materials resemble rocks and gemstones rather than self-regulating and adaptive cells.
Luckily, there is more to life than thermodynamic equilibrium. In fact, all living beings rely on self-assembled structures that form or operate far from their thermodynamic ground state. To function, such systems rely on the continuous net exchange of energy or matter with the environment. They are therefore termed dissipative. 5 The dependence on an influx of energy endows the materials with a direct way of interacting with their environment and leads to the fascinating behavior that underlies the transport, motility, and proliferation of cells. 6
Inspired by that functional richness, the colloidal science community is venturing into the relatively unexplored territories of out-of-equilibrium systems. To imprint active behavior onto colloids, one can follow multiple strategies. For example, external magnetic or electric fields can be used to induce a polarization or magnetization of the particles that triggers their assembly. As soon as the field is removed, the driving force for structuration vanishes and the assemblies disintegrate. Fascinating dynamic structures have been obtained through the temporal control over the strength and direction of the applied fields and over the field susceptibility of the particles. 7
Alternatively, one can rely on particles that actively propel themselves (see “Microswimmers with no moving parts” by Jeffrey Moran and Jonathan Posner, Physics Today, May 2019, page 44
In this article we focus instead on a new and different class of out-of-equilibrium colloidal systems. Akin to examples from the biological world, such systems rely on molecular fuels or light to drive their assembly. 8
Microtubules
To illustrate the hallmarks of fuel-driven systems, we take microtubules as an archetypal example. 9 Microtubules are a key structural element of the cytoskeleton, and they are able to reshape and remodel themselves in response to triggers from the outside world. They make highly complex cellular behavior possible.
The tubular structures are formed via the assembly of tubulin dimers. Each tubulin dimer comprises an α tubulin segment and a β tubulin segment, both of which can bind to guanosine triphosphate (GTP). The molecule is an energy-rich fuel, which promotes the tubulin dimers into a self-assembling state (see figure
In essence, the disassembly of microtubules is driven by the conversion of GTP (fuel) to GDP (waste). Only if the addition of activated tubulin outcompetes the fuel-to-waste conversion in the end caps can fibers be formed. Because the structures’ formation is governed by the kinetics of both tubulin attachment and fuel-to-waste conversion, microtubules are extremely dynamic structures that are able to respond to small fluctuations in the local fuel concentration.
The complete biomolecular machinery involved in regulating the behavior of microtubules is even more delicate and complex than outlined here. Nevertheless, one can discern the basic requirements to imprint its dynamic features on synthetic colloidal materials. Of particular importance is the need to establish a direct link between energy-consuming networks and the assembly process. 5
The link is depicted in figure
The crucial contrast with the self-assembled structures in equilibrium is that assemblies formed after activation occupy energy states far from the global free-energy minimum. When the fuel runs out, the rate of particle deactivation outcompetes the rate of activation and inactive, non-assembling particles accumulate. Eventually, the assembly disintegrates. The fuel dependence ensures that the assembly behavior of those out-of-equilibrium systems is governed by reaction kinetics rather than thermodynamics, which is the case for equilibrium processes.
Interestingly, the fuel-driven assembly cycle depicted in figure
Despite their generality, the rules of out-of-equilibrium design are tricky to transfer to the colloidal domain. Conceptually, nothing changes. Handling building blocks with colloidal dimensions, however, requires deft control over the interactions between the particles. The interactions can be embodied in the interparticle potential U, which is the sum of repulsive and attractive contributions. Van der Waals forces, which are almost always present, contribute to the attractive part of U. 1 They arise from a mismatch in polarizability of the particles and their surrounding medium.
Because their magnitude scales with particle size, van der Waals forces are much more pronounced for colloidal particles than they are for their molecular counterparts. To prevent strong clustering of colloids, the attractions need to be overcompensated with repulsions. In traditional colloidal systems, the goal is achieved by decorating the surface of the particles with charged moieties or polymer brushes. 1 The resulting electrostatic or steric stabilization ensures the particles remain as well-dispersed single entities in their surrounding medium.
To imprint fuel-responsive behavior on a colloidal system, the fuel needs to temporally switch U from net repulsive to slightly attractive. “Slightly” is crucial here. When the fuel generates attractions that are too strong, the colloidal bonds formed in the activated state are likely irreversible—again because of van der Waals forces, which are extremely strong at short interparticle distances. Once trapped in those deep potential minima, colloids will never—or at least on reasonable time scales—redisperse. Any dynamic, fuel-dependent clustering characteristics are lost.
An additional difficulty associated with the colloidal size of the building blocks is the mismatch in dynamics. Colloidal objects are significantly more sluggish than molecules. When using molecular fuels to guide the assembly of those slower particles, the deactivation reaction’s kinetics have to be tuned to keep the particles in the activated state long enough for them to meet each other via slow, diffusive processes. If deactivation precedes encounters, assembly is forestalled, and all supplied energy will be wasted. Bridging those length and time scales becomes increasingly challenging for ever-larger particles. For that reason, most systems reported to date involve relatively small nanoparticles. 11
Because of those additional challenges, the first generation of out-of-equilibrium colloidal materials started to appear only in the last few years. It goes beyond the scope of this article to discuss all system designs out there. If you want to learn more, please refer to our 2020 review. 11 For a flavor of the state of the art, we highlight two archetypal classes of systems. Each is characterized by the nature of their fuel source.
Small molecules as fuels
Directly inspired by microtubules and other biological systems, some synthetic colloidal systems run on molecular fuels. An example reported by one of us (van Ravensteijn), Wouter Hendriksen, and our collaborators illustrates the use of molecular fuels to transiently affect colloidal interactions in a striking way (see figure
Figure 4.

Colloidal assembly can be sustained by molecular fuel or by light. In the molecular case (a), the equilibrium state (blue) corresponds to the building blocks being charged, stable, and disassembled. Fuel converts the charged moieties to hydrophobic neutral groups (red) and activates them. Once activated, the particles cluster. The hydrophobic groups are not stable in the reaction mixture and slowly convert back to their negatively charged form. Buildup of charges on the particles leads to cluster disintegration. (Adapted from ref.
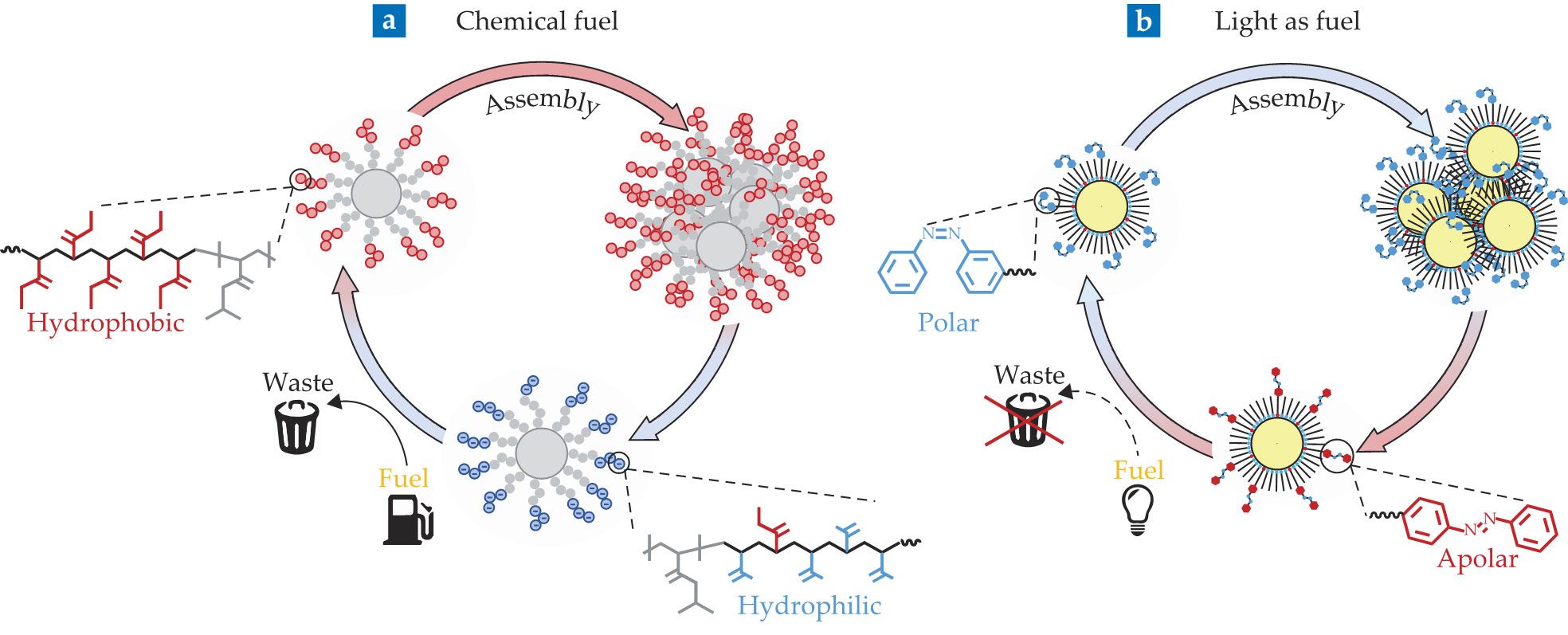
But when fuel is added, the negatively charged groups are converted to neutral, hydrophobic moieties (red in figure
The cycle cannot be repeated indefinitely because of the accumulation of waste products, which are poisonous to the system. If they remain, they cause irreversible damage to the reaction network by dissolving particles, cleaving nonfuel related bonds, and so on. As soon as the waste is removed, however, new fuel-driven cycles can resume. Waste removal can be achieved through continuous flow, through membrane reactors, or with fuels that generate waste products that spontaneously withdraw themselves from the system through evaporation or precipitation. 13 Biology solved the problem of waste accumulation with elegant regulating mechanisms that use molecular pumps and the coupling of several reaction cycles in tandem. Analogous strategies in the synthetic world could yield materials with extended lifetimes.
Job Boekhoven of the Technical University of Munich and his coworkers have developed a robust, fuel-driven system. In it, precursor particles carry charged groups on their surface that could be temporarily neutralized by the addition of a chemical fuel. 14 The authors showed that different assembled states could be reached depending on the fuel concentration and on the way fuel is supplied over time. That the properties of the final system depend on the fuel’s processing or history is unique to out-of-equilibrium materials.
Light as fuel
Using light to supply energy to dissipative colloidal systems is an alternative strategy. Compared with molecular fuels, using light—or, more precisely, photons—circumvents waste accumulation, thereby boosting the number of assembly cycles that can be performed.
Archetypal light-driven systems rely on photo-induced conformational changes of stabilizing ligands that are immobilized on the surface of the particles.
15
An example of particles functionalized by azobenzene appears in figure
Relying on light as a fuel also provides a way to regulate where the assembly occurs, because light can be delivered with high spatial resolution. That advantage was neatly illustrated by Bartosz Grzybowski and his colleagues, who fabricated self-erasing images composed of gold nanoparticles. 15 By projecting an image onto a substrate that contained azobenzene-functionalized particles, the researchers induced assembly exclusively in the illuminated areas. Because the nanoparticles are plasmonic in character, their color changes on assembly. And because the color change is limited to the illuminated areas, an image can be created. Being sustained by light, the assembled state is metastable; the image slowly fades and erases itself.
Into the future
Variations on the two exemplar systems—molecule fueled and light fueled—are being developed at a rapid pace and illustrate the robustness and versatility of the general approach. Of especial interest are systems whose particles are not actively participating in the reaction cycle but respond to a reaction network that runs in the background. Although those reaction cycles are also fueled by small molecules or light-switchable compounds, the tactic eliminates the need to modify the surfaces of colloidal particles through what are often complex synthetic procedures. Reaction cycles that reversibly change pH or surface tension of depletants are just two examples. 11 Additionally, hybrid bio–synthetic systems that combine biomolecular fuel cycles and the tunability of enzymatic or DNA-mediated reaction networks with manmade colloids present a promising future direction. 16
With those synthetic principles and design rules in hand, it is now up to us and others in the field to start exploring what dissipative colloidal systems can really do. The use of fuels is a promising route to transition from colloidal rocks to colloidal cells. Analogous to the role hard colloidal particles played in elucidating the physics underlying atomic crystallization, the colloidal systems might shed light on the fundamentals of biomolecular out-of-equilibrium processes. What’s more, by controlling the interplay between reaction kinetics and particle assembly, researchers can obtain materials that have not just colloidal characteristics but also lifelike ones. Imagine colloidal structures that are self-healing when damaged or that display dynamic oscillating behavior when coupled to periodic oscillations in fuel concentrations.
Toggling between repulsive and attractive states was recently proposed as a route to generate colloidal structures not attainable by straightforward equilibrium self-assembly. 17 Reaching that level of control will entail regulating the relative orientation of the particles in the assembled state and finding fuel reaction networks that perfectly match the colloidal dynamics.
In light of the fast pace by which the field is developing, we have no doubt that those hurdles will be surmounted soon, and we will witness a dynamic revolution in colloidal science.
References
1. D. H. Everett, Basic Principles of Colloid Science, Royal Society of Chemistry (1988).
2. A. Einstein, Ann. Phys. (Leipzig) 322, 549 (1905). https://doi.org/10.1002/andp.19053220806
3. L. J. Bonderer, A. R. Studart, L. J. Gauckler, Science 319, 1069 (2008). https://doi.org/10.1126/science.1148726
4. N. Vogel et al., Chem. Rev. 115, 6265 (2015). https://doi.org/10.1021/cr400081d
5. G. Ragazzon, L. J. Prins, Nat. Nanotechnol. 13, 882 (2018). https://doi.org/10.1038/s41565-018-0250-8
6. E. Karsenti, Nat. Rev. Mol. Cell Biol. 9, 255 (2008). https://doi.org/10.1038/nrm2357
7. V. Liljeström et al., Curr. Opin. Colloid Interface Sci. 40, 25 (2019). https://doi.org/10.1016/j.cocis.2018.10.008
8. K. Das, L. Gabrielli, L. J. Prins, Angew. Chem. Int. Ed. 60, 20120 (2021). https://doi.org/10.1002/anie.202100274
9. A. Desai, T. J. Mitchison, Annu. Rev. Cell Dev. Biol. 13, 83 (1997). https://doi.org/10.1146/annurev.cellbio.13.1.83
10. S. A. P. van Rossum et al., Chem. Soc. Rev. 46, 5519 (2017). https://doi.org/10.1039/C7CS00246G
11. B. G. P. van Ravensteijn et al., Langmuir 36, 10639 (2020). https://doi.org/10.1021/acs.langmuir.0c01763
12. B. G. P. van Ravensteijn et al., J. Am. Chem. Soc. 139, 9763 (2017). https://doi.org/10.1021/jacs.7b03263
13. N. Singh et al., Adv. Mater. 32, 1906834 (2020). https://doi.org/10.1002/adma.201906834
14. R. K. Grötsch et al., J. Am. Chem. Soc. 141, 9872 (2019). https://doi.org/10.1021/jacs.9b02004
15. R. Klajn et al., Angew. Chem. Int. Ed. 48, 7035 (2009). https://doi.org/10.1002/anie.200901119
16. J. Deng, A. Walther, Adv. Mater. 32, 2002629 (2020). https://doi.org/10.1002/adma.202002629
17. M. Tagliazucchi, E. A. Weiss, I. Szleifer, Proc. Natl. Acad. Sci. USA 111, 9751 (2014). https://doi.org/10.1073/pnas.1406122111
More about the authors
Bas van Ravensteijn is an assistant professor in the department of pharmaceutics, Utrecht Institute for Pharmaceutical Sciences, at Utrecht University in the Netherlands. Ilja Voets leads the Laboratory of Self-Organizing Soft Matter in the department of chemical engineering and chemistry and is a member of the Institute for Complex Molecular Systems at Eindhoven University of Technology, also in the Netherlands.






