Clathrate hydrates under pressure
DOI: 10.1063/1.2800096
When water comes in contact with simple gases and hydrocarbon molecules at ambient conditions, little happens. But at high pressure and low temperature, an icelike framework of hydrogen bonds can build polyhedral cages around guest molecules like methane, nitrogen, and argon to form clathrate hydrates (see
Since their identification in Michigan’s natural-gas lines in 1934, clathrates have been recognized as the largest hindrance to flow in offshore gas and oil pipelines, which supply much of the world’s energy. 1 Pipelines that run along the ocean floor and through arctic regions are especially prone to the sort of clathrate blockages shown in figure 1, which can create serious safety and economic problems. To manage the problems, engineers have recently shifted from using expensive antifreezes like alcohols or glycols that are mixed with the natural gases to using low doses of chemicals such as surfactants that allow clathrates to form but not block the flowlines. The clathrates can then pass harmlessly through as a cold slurry of nonagglomerating particles.

Figure 1. A clathrate plug recovered from an offshore gas flowline. The clathrate is metastable at ambient conditions and dissociates to release its natural gases over several hours.
(Courtesy of Petrobras.)

The compounds form naturally in Earth’s permafrost and continental shelves. The largest source of clathrates is the oceans, however, where they are highly dispersed and trapped in layers of marine sediment. Indeed, methane clathrates are the most abundant form of hydrocarbon on our planet, with some estimates putting their mass at twice that of Earth’s other hydrocarbons combined.
1
Clathrates may also be quite prevalent on other planetary bodies in our solar system. Evidence points to the existence of carbon dioxide and methane clathrates on Mars, in Halley’s comet, and in Titan and Enceladus, two of Saturn’s icy moons, where thermodynamic conditions favor their formation.
2
Two years ago the Cassini spacecraft measured spectacular plumes of hydrocarbon gases mixed with water-ice spewed from the surface of Enceladus, for instance (see
Although pressure has long been an important variable in investigations of clathrates, it was thought as recently as the 1990s that their structures would not survive significant compression, and research was limited to fairly moderate pressures—typically below a few hundred megapascals—until the late 20th century. More recently, multiple new phases of clathrates and other simple molecular solids have been discovered by squeezing water and gases in diamond anvil cells to pressures well above a gigapascal. The research has extended our understanding of the interiors of the gas planets and their satellites, in which clathrates, and molecular solids more generally, could be major constituents. The synthesis of new materials is likely to have an impact on both fundamental science and applied technology.
Molecules trapped in ice cages
Clathrate hydrates are inclusion compounds in which a hydrogen-bonded water framework—the host lattice—traps “guest” molecules (typically gases) within ice cages. The gas and water don’t chemically bond, but interact through weak van der Waals forces, with each gas molecule—or cluster of molecules in some cases—confined to a single cage. The interactions lower the free energy of the gas–water system and ensure the compound’s stability over a range of temperatures and pressures, provided a sufficient number of cages are filled.
The clathrates typically crystallize into one of the three main structures illustrated here. Structure I is composed of two types of cages: dodecahedra, 20 water molecules arranged to form 12 pentagonal faces (designated 512), and tetrakaidecahedra, 24 water molecules that form 12 pentagonal faces and 2 hexagonal ones (51262). Two 512 cages and six 51262 cages combine to form the unit cell. The pictured structure I illustrates the water framework and trapped guest molecules.
Like structure I, structure II has a cubic unit cell with two types of cages. (For simplicity, the guest molecules are not shown.) In total, 16 dodecahedra (512) and 8 hexakaidecahedra, 12 pentagonal faces and 4 hexagonal faces (51264), combine to form the unit cell. Structure H, discovered by John Ripmeester and collaborators in 1987, occurs more rarely. Its unit cell is hexagonal with three types of cages. It contains three small pentagonal dodecahedra (512), two medium irregular dodecahedra (435663), and one icosahedron (51268).
The size of available guest molecules largely determines the clathrate structure that eventually forms. The fit of the guest molecules within the various cages determines the pressure and temperature at which the structure is stable. Small molecules like argon, krypton, and nitrogen fill the small 512 spaces in a way that optimizes the van der Waals forces between the guest and the water framework.
Because there are twice as many small cages as large ones in structure II, small molecules preferentially prefere to crystallize within a structure II clathrate. Conversely, molecules such as methane, hydrogen sulfide, and carbon dioxide stabilize the small 512 and large 51262 cavities of a structure I clathrate. Larger molecules such as propane and i-butane can fill the larger of the two types of cavity in a structure II clathrate. Molecules that are larger still—typically larger than 7.3 Å, such as methylcyclohexane and adamantane—can only fit into the icosahedral cavity of structure H and require a second guest such as methane to stabilize the two small (512 and 435663) cages of that clathrate structure. Small molecules such as He2, Ne2, and H2 are only contained within a clathrate hydrate framework at high pressures, usually above 200 MPa at room temperature.

Mining methane
The tremendous amount of methane clathrates residing in Earth’s continental margins and polar regions makes them a tempting natural resource (see figure 2). With anecdotal exceptions, most of the hydrocarbons in natural clathrates come from cold, biogenic sources—that is, bacterially generated methane—rather than the deeper, warm, thermogenic sources of more common oil and natural gas. Although the total mass of the clathrate reservoir in the oceans may be an order of magnitude greater than what lies buried under permafrost, the presence of very high concentrations of clathrates in the accessible permafrost makes it a more attractive target.

Figure 2. Fire and ice. A chunk of methane clathrate displays its potential as an energy source. As the compound melts, released gas feeds the flame and the ice framework drips off as liquid water.
(Courtesy of Gary Klinkhammer, Oregon State University.)

Indeed, sweet spots in the permafrost were the first to be explored. The Messoyakha field in Siberia, explored in the 1970s, and the Mallik well drilled in Canada in 2002 are two proof-of-concept examples; gas was recovered from clathrates by injecting hot water or depressurizing the area to melt the clathrate framework and flush out the methane. Most of the clathrates identified in the extensive Mallik 2002 tests were found 300–700 m beneath the permafrost in sediments that date back to Earth’s Tertiary period some 2 million to 65 million years ago. The overlying permafrost provides an effective seal for the clathrate reservoir and makes production more viable. Even so, recovering clathrates using a method that can succeed commercially remains elusive. It also remains to be seen whether recovery from marine reservoirs can be economically viable.
As a greenhouse gas, methane is approximately twenty times more potent than carbon dioxide. And its release into the atmosphere could profoundly affect Earth’s climate. Large-scale dissociation of clathrates may be associated with previous global warming events such as the late Paleocene temperature maximum around 55.5 million years ago, 3 when ocean surface temperatures rose between 5 and 8 degrees and worldwide extinctions of marine life occurred. The contribution of clathrate decomposition to the global methane budget remains an important unknown, 4 and the sensitivity of clathrate deposits to climate changes and warming trends awaits further research.
The dynamics of clathrates on Enceladus
In July 2005 the Cassini spacecraft flew within 170 km of Saturn’s moon Enceladus, revealing near the south pole a unique landscape littered with house-sized boulders and tectonic features.
18
Those features include what have been named “tiger stripes,” 130-km-long surface fractures that straddle the south pole, pictured
Researchers originally proposed that the plumes tapped shallow pockets of liquid water in a water-ice shell—the “Cold Faithful” model. Last year Susan Kieffer (University of Illinois at Urbana-Champaign) and colleagues hypothesized a more sophisticated model—dubbed “Frigid Faithful”—to account for the concentrations of CO2, CH4, and other gases observed by Cassini. 17 In that model, the plumes erupt from a reservoir of clathrate hydrates mixed with water-ice. The solubility of hydrocarbons in clathrates is enormous compared to their solubility in liquid water and helps explain the concentrations observed in the plumes. The 10:1 ratio of water vapor to hydrocarbon gases in the plume is similar to the ratio, typically 7:1, of water to guest molecules in a clathrate.
Kieffer, Gustavo Gioia, and their colleagues have now extended the model to account for the origin of Enceladus’s tectonic features. 18 A heat source deep under the south pole, they argue, produced a temperature gradient in the surrounding material early in the moon’s history. The resulting tensile stresses expanded the clathrate reservoir, which eventually cracked and developed the four large tiger-stripe fractures, extending 35 km into the moon. The gigantic radial rifts and ring of ridges around the stripes formed from similar stresses. The plumes erupt where the fractures expose and decompress some clathrates in the reservoir. Released gases rush up the tiger stripes, transporting heat and matter toward the surface.


The mechanism by which clathrates form in sediments can have a significant impact on the mechanical strength of those sediments. But it’s a complicated issue. The strength of the sediment depends on the concentration and distribution of gas dissolved in the water that fills the pores between sediment grains. If the gas is highly concentrated, then a large amount of clathrates will form and the sediment grains are likely to be cemented together; with lower amounts of gas, the clathrates will fill the pores without cementing the grains.
Assessing the mechanical strength of clathrate deposits is important in evaluating their role in global climate change. One key concern is the effect of natural gas production, including equipment such as wellbores, platforms, and flow-lines, whose weight sits on clathrate-bearing sediments and can impact the mechanical stability of the sediments. Numerical simulations by George Moridis at Lawrence Berkeley National Laboratory suggest that local heating—as when warm reservoir fluids are transmitted through uninsulated pipes—can significantly affect that stability by triggering the dissociation of gases within the clathrates. The weight of gas-production platforms and equipment may play a role if sediments are unconsolidated and compressible. Conversely, the simulations of strong sediments showed virtually no adverse effect from the heavy platforms. Indeed, the weight of structures on the ocean floor may actually further pressurize the clathrates and enhance their mechanical stability.
Finding the right fit
To assess possible environmental impacts and explore the technological potential of clathrates, one needs to start with an understanding of its novel physics and chemistry. Consider the simple model of a spherical, free-rotating guest molecule that fills a polyhedral cage whose bars are made of hydrogen bonds. Pressure can induce changes in the structure as both cage and guest are compressed, and thus allows Nature to tailor both for an improved fit. As free space becomes scarce during increased compression, the ice framework can reorganize itself into different types of cages to accommodate the guest. And that reorganization prompts the transition to different types of clathrate structures (see
There is a limit to how efficiently guest molecules can fill the clathrate cages, and conventional wisdom suggests that at high pressures the relatively open clathrate structures should collapse, leaving a mixture of pure ice and solute. But Nature has something more complicated up her sleeve. The high pressures can force the H2O frameworks to reorganize themselves into one of the dense phases of pure ice. Ice exhibits a rich array of at least 15 stable and metastable crystalline and amorphous phases, and many of the resulting structures contain small voids that can act as cages for small guest molecules. In the first discovery of such filled ices, helium atoms were found to sit in the cages in a rhombohedral ice II framework. 6 Molecular hydrogen forms a filled ice II structure as well. But at pressures above 2 GPa, the ice framework changes, with the hydrogen molecules filling cages in a cubic ice Ic structure. 7 The large number of small cages in the ice Ic structure allows it to accommodate one H2 for each H2O molecule, which leads to a guest-to-host ratio six times higher than that of typical clathrate structures. Work on methane-filled ices demonstrates that they can be stable to extremely high pressures. 8
Filling efficiency may be improved without altering the cage configuration. Pressure can induce more than one guest molecule to squeeze into a single cage. In 1997 Werner Kuhs of the University of Göttingen found that under compression, the number of nitrogen molecules in the large cages of structure II clathrate may be, on average, more than one. A dramatic example of multiple occupancy is found in the hydrogen–water system, in which a structure II clathrate forms at 200 MPa and 140 K. Four hydrogen molecules combine to form a tetrahedral cluster that fills and stabilizes the large cages. 9
The behavior of the molecular clusters in clathrates is fertile ground for studying novel molecular interactions. The manner in which the cages hold the clusters in the hydrogen clathrate is quite intriguing. Contrary to the view that the guests are trapped because they are too large to escape through the space between cage bars, the hydrogen molecules are small and free to move around. Theoretical analysis and neutron diffraction experiments confirm that the individual hydrogen molecules and the four-molecule clusters freely rotate inside their cages. The dispersive interactions between the hydrogen clusters and the ice framework stabilize the structure and actually cause the rapidly rotating molecules to cling onto the bars and remain in the cage if the conditions are right. 10
Hydrogen-filled ices also display novel behavior. A recent study using nuclear magnetic resonance showed that those compounds exhibit extremely fast, liquidlike diffusion of the hydrogen guests through the ice framework. 11 That is, although the guests prefer to remain caged, they are free to diffuse between cages.
Clean energy
One of the main hurdles to a hydrogen economy is finding a practical hydrogen storage material for mobile applications (see reference and the article by George Crabtree, Mildred Dresselhaus, and Michelle Buchanan in Physics Today, December 2004, page 39
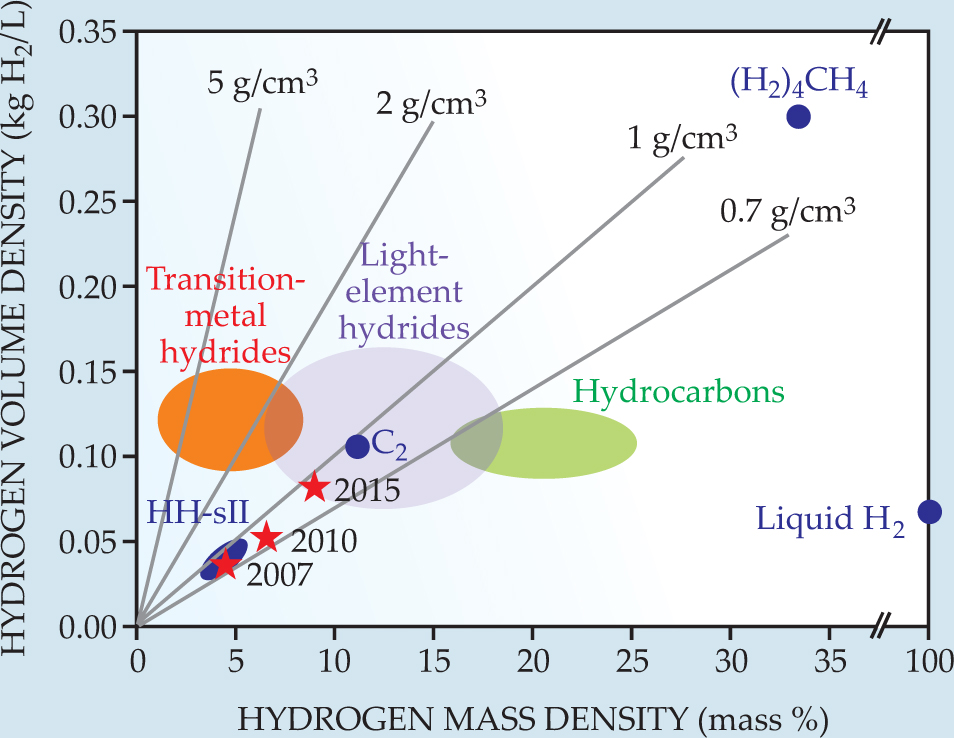
Many of the unique properties of hydrogen–water compounds make their phases attractive candidates as storage materials: Unlike batteries, compressed gas, and fossil fuels, clathrates are clean, safe, and, in principle, cheap. Their storage density, however, falls short of the US Department of Energy’s target goals for the next few years (see figure 3). Filled ice Ic is hydrogen rich—capable of holding 11.2% of its weight as molecular hydrogen, not including the hydrogen bound up in the ice framework. Once its stored molecular hydrogen is consumed—oxidized in a fuel cell, say—the only byproduct is water. The fast diffusion of the hydrogen molecules and their weak bonding with the host lattices facilitate the release and reabsorption of hydrogen.

Figure 3. The hydrogen storage density in structure II hydrogen hydrate (HH-sII), hydrogen-filled ice Ic (C2), the hydrogen–methane molecular compound (H2)4CH4, and various chemical compounds is plotted as a function of the hydrogen mass fraction. The straight lines indicate the total density of the storage material. Red stars show US Department of Energy target densities for the years 2007, 2010, and 2015 for an onboard hydrogen-storage system. Those densities refer to the entire system, including the tank and any refrigeration equipment. The figure illustrates the tremendous promise of (H2)4CH4.
(Adapted from F. Schüth,
However, before we start imagining fueling our automobiles with hydrogen-filled ice cubes rather than gasoline, we must envision solutions to some major obstacles. The primary one is the high pressures needed to stabilize the phases of clathrates and filled ices. In terms of storage, clathrates can display a large range of metastability. Once formed, under 200 MPa pressure at 140 K, the H2 clathrates can be stored indefinitely at ambient pressure, provided the temperature is kept below 110 K. Similarly, a filled ice crystallized in the Ic structure can be stored at one-tenth of its room-temperature synthesis pressure—down to 200 MPa at liquid-nitrogen temperature.
For practical production, one needs to be able to synthesize the hydrogen storage material at more moderate conditions. Adding a molecule like tetrahydrofuran (C4H8O) stabilizes the structure II clathrates at near-ambient pressures and temperatures. But it also significantly reduces the hydrogen-storage capacity because the THF molecules fill the large cages. 13 Studies that investigate ways to enhance the stability of clathrates and filled ices represent a promising approach for finding new hydrogen storage materials. Once hydrogen-rich phases are found at high pressure, researchers can search for a chemical pathway to stabilize the structure near ambient conditions.
The broader view
Clathrate hydrates belong to a broad class of materials known as molecular solids, which are formed of atoms or molecules held together by weak intermolecular forces produced by dipole–dipole interactions and hydrogen bonds. Pressure increases possible bonding configurations between atoms, and experiments that force molecules together have led to the discovery of a significant number of novel molecular solids. About fifteen years ago researchers found that helium and molecular nitrogen, both inert gases at ambient pressure, form the molecular solid He(N2)11 when subjected to high pressure. Compounds of neon and helium, NeHe2, and of argon and hydrogen, Ar(H2)2, were reported shortly thereafter. 14
In the methane–hydrogen system under pressure, there are no less than four stoichiometric crystalline compounds that consist of freely rotating methane and hydrogen molecules; each compound contains a different ratio of methane to molecular hydrogen. 15 One of those compounds, (H2)4CH4 or H4M, contains a higher hydrogen mass density than any known material except pure hydrogen itself, and is thus a potential candidate for hydrogen storage (see figure 3). With four hydrogen molecules for each methane, the material contains a whopping 33.4% molecular hydrogen by mass, or 50% total hydrogen if one includes the four hydrogen atoms within each methane molecule. The methane could also be used as fuel along with the hydrogen content in the material.
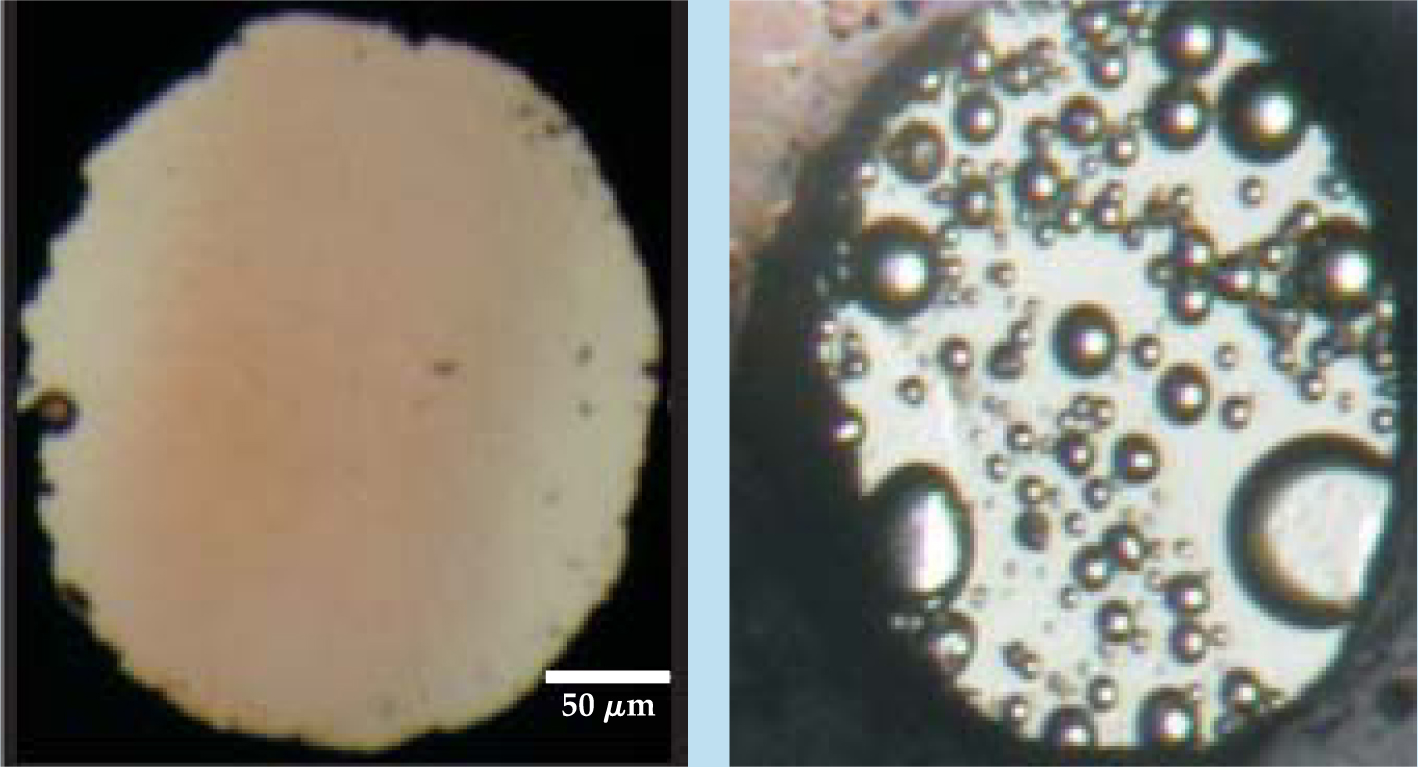
Cryogenic experiments have further shown that H4M is stable over a wide range of pressures and temperatures. One can afford to give up a significant fraction of the enormous hydrogen storage capacity in the material in the search for a chemical pathway that stabilizes the structure close to ambient conditions. Adding a molecule such as THF might do the trick. Other approaches may facilitate the synthesis of novel and simple molecular compounds. Last year one of us (Mao) along with colleagues from the Carnegie Institution of Washington, the National Synchrotron Radiation Research Center in Taiwan, and the University of Chicago found that x rays can split the oxygen–hydrogen bonds in water-ice held at pressures above 2.5 GPa and stabilize a molecular alloy composed of H2 and O2 (see figure 4). 16 That new compound can then be stored at high pressure without decomposing back into ice.

Figure 4. These photographs of water compressed in a diamond anvil cell illustrate the effect of x ray irradiation on a sample crushed under 9 GPa of pressure: the formation of an O2–H2 alloy. (a) The light brown streak running through the middle of the cell marks the portion irradiated by an x-ray beam. The small ruby ball at left was used for pressure calibration. (b) As pressure from the cell is reduced below 1 GPa, bubbles of molecular oxygen and hydrogen are released from the newly formed compound.
(Adapted from ref. 16.)
We have barely scratched the surface of what has already proven to be a fruitful field for both fundamental physics and materials applications. Among the many questions researchers are likely to explore as they attempt to discover new molecular solids, two broad ones loom large: What are the rules that control the synthesis of clathrates and hydrogen-rich molecular solids at high pressure and their recovery at low pressure? And can those rules be creatively applied to designing materials in the service of various environmental and energy applications?
We thank Russell Hemley, Ho-kwang Mao, and John Tse for their helpful comments.
References
1. E. D. Sloan, C. A. Koh, Clathrate Hydrates of Natural Gases, CRC Press/Taylor & Francis, Boca Raton, FL (2008).
2. J. S. Loveday et al., Nature 410, 661 (2001); https://doi.org/10.1038/35070513
C. C. Porco et al., Science 311, 1393 (2006). https://doi.org/10.1126/science.11230133. G. R. Dickens, Science 299, 1017 (2003). https://doi.org/10.1126/science.1080789
4. W. S. Reeburgh, Chem. Rev. 107, 486 (2007). https://doi.org/10.1021/cr050362v
5. I. -M. Chou et al., Proc. Natl. Acad. Sci. USA 97, 13484 (2000). https://doi.org/10.1073/pnas.250466497
6. D. Londono, W. F. Kuhs, J. L. Finney, Nature 332, 141 (1988). https://doi.org/10.1038/332141a0
7. W. L. Vos et al., Phys. Rev. Lett. 71, 3150 (1993). https://doi.org/10.1103/PhysRevLett.71.3150
8. J. S. Loveday et al., Phys. Rev. Lett. 87, 215501 (2001); https://doi.org/10.1103/PhysRevLett.87.215501
S. Machida et al., Phys. Earth Planet. Inter. 155, 170 (2006). https://doi.org/10.1016/j.pepi.2005.12.0089. W. L. Mao et al., Science 297, 2247 (2002); https://doi.org/10.1126/science.1075394
K. A. Lokshin et al., Phys. Rev. Lett. 93, 125503 (2004). https://doi.org/10.1103/PhysRevLett.93.12550310. S. Patchkovskii, J. S. Tse, Proc. Natl. Acad. Sci. USA 100, 14645 (2003). https://doi.org/10.1073/pnas.2430913100
11. T. Okuchi et al., Phys. Rev. B 75, 144104 (2007). https://doi.org/10.1103/PhysRevB.75.144104
12. L. Schlapbach, A. Züttel, Nature 414, 353 (2001). https://doi.org/10.1038/35104634
13. L. J. Florusse et al., Science 306, 469 (2004); https://doi.org/10.1126/science.1102076
H. Lee et al., Nature 434, 743 (2005). https://doi.org/10.1038/nature0345714. W. L. Vos et al., Nature 358, 46 (1992); https://doi.org/10.1038/358046a0
P. Loubeyre et al., Phys. Rev. Lett. 70, 178 (1993); https://doi.org/10.1103/PhysRevLett.70.178
P. Loubeyre, R. Letoullec, J. -P. Pinceaux, Phys. Rev. Lett. 72, 1360 (1994). https://doi.org/10.1103/PhysRevLett.72.136015. M. S. Somayazulu et al., Science 271, 1400 (1996). https://doi.org/10.1126/science.271.5254.1400
16. W. L. Mao et al., Science 314, 636 (2006). https://doi.org/10.1126/science.1132884
17. S. W. Kieffer et al., Science 314, 1764 (2006). https://doi.org/10.1126/science.1133519
18. G. Gioia et al., Proc. Natl. Acad. Sci. USA 104, 13578 (2007). https://doi.org/10.1073/pnas.0706018104
More about the authors
Wendy Mao is an assistant professor with joint appointments in the photon science department at SLAC and the department of geological and environmental sciences at Stanford University in California. Carolyn Koh is an associate professor and Dendy Sloan the Weaver Distinguished Professor in the department of chemical engineering at the Colorado School of Mines in Golden, where they codirect the Center for Hydrate Research.
Wendy L. Mao, 1 Stanford University, California, US .
Carolyn A. Koh, 2 Colorado School of Mines, Golden, Center for Hydrate Research, US .
E. Dendy Sloan, 3 Colorado School of Mines, Golden, Center for Hydrate Research, US .




