Circulating tumor cells: Cancer’s deadly couriers
DOI: 10.1063/PT.3.2275
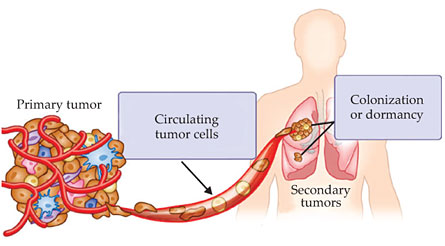
Cancer is one of the most lethal diseases in the world. It is caused by an accumulation of genomic mutations, deletions, or amplifications that lead cells to divide uncontrollably and invade normal tissue. Eventually, some cells may detach from the primary tumor, pass through neighboring tissues, and make their way into the bloodstream. Those circulating tumor cells (CTCs) are transported from the primary tumor to distant sites such as bone, lung, liver, or brain, where they can form secondary tumors in a process known as metastasis. That spread of cancer cells, illustrated in figure 1, accounts for more than 90% of cancer-related deaths. 1

Figure 1. Circulating tumor cells (CTCs) detach from the primary tumor and travel through the bloodstream to distant sites, where they can form secondary tumors. CTCs can consist of diverse subpopulations of cells including epithelial cells, which resemble those found in tissues where cells are well adhered to one another and behave collectively; mesenchymal cells, which behave more individualistically; epithelial–mesenchymal transition cells; and cancer stem cells. (Adapted from A. Farrell, Nat. Med.17, 262, 2011,
The first reported sighting of CTCs was in 1869, when Australian pathologist Thomas Ashworth observed some cells in the blood of a metastatic cancer patient that did not resemble blood cells. Rather, they looked similar to those found in the solid tumors. 2 It’s now known that CTCs can travel through the circulatory system, either as single cells or as clusters called microemboli, before being lodged in a distant organ site.
Fortunately, metastasis is an inefficient process, and most CTCs don’t go on to form secondary tumors. 3 Instead, some undergo apoptosis, or programmed cell death, once outside their normal environment. Others are broken up into fragments by the physical forces acting on them as they journey through the harsh and demanding circulatory system. Still other CTCs are killed by the body’s immune system. Furthermore, certain cancer types can spread to and survive at only specific organ sites. Whatever their destiny, CTCs hold important information about the tumor from which they came. That information may offer a new route to cancer diagnosis and treatment.
Toward a liquid biopsy
At present, cancer is usually diagnosed via surgical removal or tumor biopsy, in which cells are removed from the tumor by fine-needle aspiration, and examined to determine their malignancy and other characteristics. That approach has several drawbacks. Biopsy is an invasive and painful process that cannot be performed repeatedly to track how cancer cells are evolving, changing, or responding to treatment. In some cases, such as advanced-stage non-small-cell lung cancer, the size and location of a tumor may be such that biopsy is not an option at all. 4 Furthermore, cancer cells can be quite heterogeneous in nature. Different secondary tumors, or even different areas of a tumor, can consist of different subpopulations of cells, some of which are benign and some malignant. Cells sampled from the tumor mass may not be representative of the characteristics or metastatic potential of the tumor.
Here then lies the potential of CTCs, which can be collected through a routine blood draw: a “liquid biopsy.” Blood can be drawn easily and frequently with little risk or inconvenience to the patient, so regular liquid biopsies would make it possible to obtain real-time information about the disease status. And because CTCs are the very cells that are undergoing metastasis—the process most likely to lead to a patient’s death—they can be more informative of disease status than cells drawn from a solid tumor.
The attractiveness of obtaining CTCs from blood has inspired cancer researchers and clinicians to propose several applications. First, CTCs may be used for early detection or screening for cancer even before the tumor can be detected or the patient reports any symptoms. Some studies have shown that in cancer-free patients, detection of CTCs can predict disease recurrence well in advance of conventional methods. 5 Highly malignant tumors, such as the aggressive skin cancer melanoma, are more likely than less malignant tumors to shed CTCs early in their growth process. 5
Second, the concentration of CTCs in the blood may be useful for prognosis. 6 Research has shown that early-stage cancer is correlated with a low CTC count and late-stage cancer with a higher count. Patients with lower CTC counts have shown significantly longer survival times compared with patients with high CTC counts. 7
Third, monitoring CTCs over the course of a treatment regime may provide rapid and accurate feedback on how the patient is responding. A declining CTC count may indicate that the patient is responding well to treatment, with tumor cells being targeted and progressively removed. On the other hand, a steady or increasing CTC number may mean that the treatment is not being effective, and the oncologist must then decide if the treatment regime should be stopped or another anticancer drug be used. 8
Fourth, for patients who have their tumors removed or have gone into remission, CTC counts may be useful in assessing if there is a recurrence. 9 A liquid biopsy once every few months could be an easy way to monitor whether the patient is disease free or is at risk of relapsing soon. 5
Isolation techniques
However, the rarity of CTCs, and the technological challenge of detecting and isolating them in blood, has hindered some of their potential uses. Typical cancer patients have between 5 and 100 CTCs per milliliter of blood, depending on the stage of cancer. Furthermore, CTCs are extremely heterogeneous, 10 with different subtypes that express different cell surface receptors—or proteins found in the cell membrane. Because cell surface receptors are often used as molecular targets for cell identification and capture, their heterogeneity in CTCs poses challenges.
Research into CTC detection and isolation has become highly interdisciplinary, involving close collaborations among clinicians, life scientists, physicists, chemists, and engineers. That is encouraging, since we need all the help we can get to combat a complex disease such as cancer. In fact, the past five years have seen a sudden proliferation of technologies to achieve more specific and sensitive detection and isolation of CTCs. We will highlight a few of the major techniques in use and in development.
One of the most prominent CTC isolation techniques involves the use of antibodies against the epithelial cell adhesion molecule (EpCAM), a protein found on the surface of some CTCs but not on red or white blood cells. The Food and Drug Administration–approved CellSearch system from Janssen Diagnostics takes that approach: EpCAM antibodies attached to magnetic nanoparticles are used to target CTCs. Once the CTCs bind to the nanoparticles, they can be pulled from the blood by a magnetic field. However, EpCAM-based techniques may miss an important class of CTCs. The very process that allows CTCs to lose their adhesion to the primary tumor and enter the bloodstream, called the epithelial–mesenchymal transition, also causes the cells to reduce their expression of EpCAM. As a result, EpCAM-expressing epithelial CTCs may not be representative of the cells most likely to form secondary tumors.
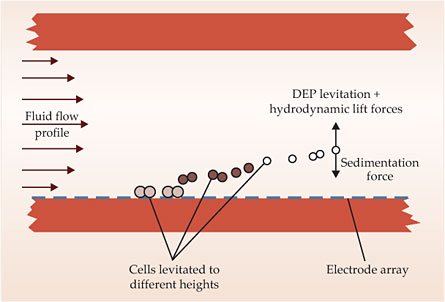
An alternative approach, developed by Peter Gascoyne and his group at the University of Texas M. D. Anderson Cancer Center, uses a technique called dielectrophoretic field-flow fractionation to isolate CTCs from blood samples. 11 The setup is shown schematically in figure 2. Under a fluid velocity gradient, different-sized cells experience different hydrodynamic lift forces. At the same time, an electrode array generates an AC electric field that induces attractive or repulsive forces on the cells, depending on the cell-membrane capacitance and the frequency of the applied field. When the frequency is chosen so that CTCs are attracted and red and white blood cells are repelled, the blood cells are propelled through the channel while the CTCs are retained. Gascoyne and his colleagues have demonstrated their technique on simulated mixtures of cancer cells in blood; a slide made from the cancer cells they separated is shown in the image on page 26.

(Adapted from ref.
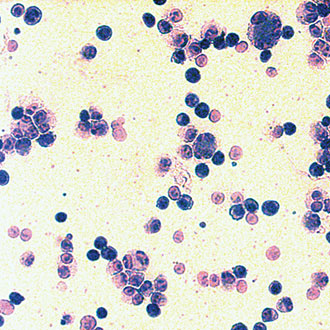

Figure 2. In dielectrophoretic field-flow fractionation, cells in a channel experience a fluid velocity gradient and an applied AC electric field. The balance of dielectrophoretic (DEP), hydrodynamic, and sedimentation forces determines the height to which a cell is levitated. With an appropriately chosen AC frequency, blood cells are washed through the channel and circulating tumor cells are left behind. (Adapted from ref.
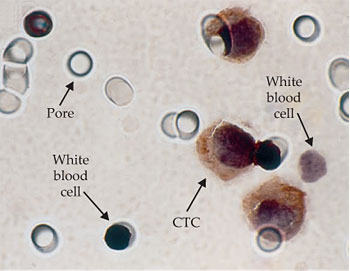
CTCs have also been isolated based purely on physical properties such as cell size and deformability. They are generally larger and stiffer than red or white blood cells, so they can be caught in a filter that allows normal blood cells to pass through. One such filtration method, developed by Patrizia Paterlini-Bréchot (INSERM in Paris) and her colleagues, is called ISET, or isolation by size of epithelial tumor cells. 12 It uses a polycarbonate membrane etched with calibrated cylindrical pores 8 µm in diameter, as shown in figure 3. In the system commercialized by Paterlini-Bréchot’s company Rarecells, 10 mL of blood is first diluted with the ISET buffer solution and then filtered using the ISET device within three minutes. ISET does not suffer from the limitations of using antibodies. However, forcing the blood through the filter creates large shear forces that can damage some of the more fragile CTCs. And retrieving CTCs from the membrane for further analysis or culture can be difficult, because they can become stuck in the pores. White blood cells, which outnumber CTCs by a factor of thousands to millions, also can get trapped in the pores.

Figure 3. Because circulating tumor cells are larger than red or white blood cells, this filter with precisely calibrated 8-µm pores can catch CTCs while allowing most blood cells to pass through. A small number of white blood cells are also caught in the filter. (Adapted from ref.
Recently researchers have begun to focus on using microfluidics for cell separation and isolation. Microfluidic methods have several advantages, including high throughput, high sensitivity, and low cost. They generally don’t rely on antibodies and allow live CTCs to be captured for analysis or culture. One of us (Lim) has led the development of several microfluidic devices, including a microfiltration biochip 13 and a spiral microfluidic biochip 14 , that isolate CTCs based on their size, deformability, and interactions as they flow through the microfluidic channels.
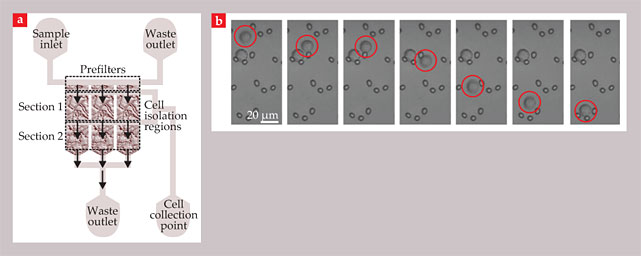
In the microfiltration biochip, shown in figure 4, micropillar arrays trap CTCs to isolate them from whole, undiluted blood. Crescent-shaped traps, each consisting of three micropillars separated by 5 µm, can selectively catch CTCs without subjecting them to the large shear forces of the ISET filter. The chip has been shown to work both on artificial mixtures of cancer cells in healthy blood and on clinical samples from lung-cancer patients.

Figure 4. A microfiltration biochip isolates circulating tumor cells by filtering them through arrays of micropillar traps. (a) The overall layout of the chip. (b) These time-sequence images show the capture of a cancer cell (circled in red). The arrangement of the traps allows cells to bypass occupied traps and prevents clogging in the device. (Adapted from ref.
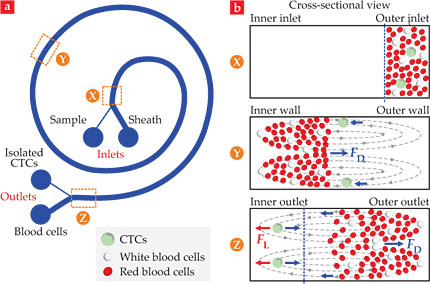
The spiral microfluidic biochip, shown in figure 5, uses an inertial separation technique called Dean flow fractionation. When fluid flows in a curved microchannel, centripetal acceleration in the radial direction can cause two symmetrical counterrotating vortices, known as Dean vortices, to form across the channel cross section. Cells flowing in the microchannel are thus pulled back and forth between the channel’s inner and outer walls. In addition to that Dean drag force, larger cells such as CTCs experience a larger inertial lift force near the inner wall. With a suitably designed channel, CTCs can be focused near the inner wall while red and white blood cells end up nearer the outer wall. The blood cells are then directed into one outlet and the CTCs into another. Both microfluidic biochips have been commercialized by Clearbridge BioMedics and are undergoing clinical tests.

Figure 5. A spiral microfluidic chip isolates circulating tumor cells (CTCs) through Dean flow fractionation. (a) A blood sample is pumped through one inlet of a curved microchannel and a sheath fluid through the other. (b) As shown in these cross-sectional views of the channel, centripetal forces set up two counterrotating Dean vortices across the channel that exert a Dean drag force FD on the cells. Because of their large size, CTCs also experience an inertial lift force FL that focuses them near the inner wall of the channel and separates them from the blood cells. (Adapted from ref.
Beyond enumeration: Every CTC counts
The discussion so far has focused on CTC count as a diagnostic and prognostic marker. But there’s a case to be made for moving beyond counting and instead focusing on analyzing specific CTCs. That is because CTC count does not always tell us a complete story when we lack critical information about the true nature of the CTCs. We know that they are biologically and genomically heterogeneous, with some being more malignant and mattering more than others. Thus we should focus on the search for biomarkers that can more accurately and definitively determine the malignant potential and biological properties of every CTC. Those biomarkers might be genes or proteins exclusively expressed in malignant CTCs. We know that aberrations of genes and proteins can influence how a cancer cell behaves or responds to treatment, and when overexpressed, they can lead to abnormal cell behavior such as uncontrolled growth or invasiveness. If we can test CTCs to see which genes and proteins have gone wrong, we can better differentiate the malignant cells from the nonmalignant ones.
We are entering an era of rapid progress in genome profiling and accumulation of sequencing data, thanks to innovative sequencing technologies such as the Next-Generation Sequencing technique. 15 Individual patients can now have their unique cancer genomes sequenced using DNA from both tumor cells and CTCs. 16 Any cancer-related variants or mutations can then be identified and the disease diagnosed.
Looking at the entire genome is important because cancer is not only about specific gene alterations but also about the way various mutations come together to give rise to the pathophysiology of the disease. One of the first comprehensive genomic profiling studies of cancer was recently performed by Jochen Geigl, Michael Speicher, and their colleagues at the Medical University of Graz in Austria, who sequenced 68 genes associated with colorectal cancer to compare mutations in the primary tumors, metastases, and CTCs in six patients. 17 Their study confirmed that mutations found in CTCs were also present in both the primary tumors and the metastases, a result that supports the potential of CTCs for tracking the changes of personal tumor genomes during progression, treatment, and relapse. In the future, the search for new definitive and confirmatory genomic biomarkers using state-of-the-art sequencing techniques will be an important priority.
Another emerging research focus is the use of CTCs in the development and administration of anticancer drugs. No drug is effective against every cancer, and matching the right drug to the right patient is a challenge. The availability of CTCs and their biomarkers can facilitate the development of therapies designed to specifically target and kill the most malignant cells, both in tumors and in the blood. One example is the antibody drug trastuzumab, marketed as Herceptin, which targets the HER2 protein that appears on the surface of breast-cancer cells. The protein normally controls breast cell growth and physiological functions. But when the gene that encodes HER2 becomes defective, it can make too many copies of itself, a process called HER2 gene amplification. The extra genes produce extra HER2 proteins, which cause the breast cells to multiply and grow uncontrollably. Many, but not all, breast cancers are associated with HER2 amplification, and Herceptin was developed specifically to target HER2-positive tumors. It’s recently been found that HER2 on CTCs is well correlated with HER2 on the cells in the primary tumor. But occasionally we find cases in which the primary tumor is HER2 negative but the CTCs are HER2 positive. 18 In those instances, Herceptin may be effective in suppressing metastasis.
During treatment, we can also continue to sample CTCs to look for biomarkers that may indicate some form of drug resistance. We can then switch to a more effective drug. By examining the different subtypes of CTCs that may elicit different responses to treatment, we can design multidrug treatment regimens to target all malignant cells and their mutations. Sampling CTCs periodically can provide real-time information for the accurate identification of the most effective therapeutic targets.
To summarize, the ease of obtaining cancer cells from a blood draw instead of a tumor biopsy makes CTCs attractive for use in diagnosis, prognosis, and treatment. But the limitations of current technologies in isolating CTCs from blood have hampered their clinical utility, because each CTC can have different properties and some important cell types may be missed by existing technologies. Researchers are working on making methods for isolating CTCs faster, more sensitive, more accurate, and affordable. In the meantime, some pertinent questions remain. How does the location of a tumor in the body influence the spatial distribution of CTCs, and what is the best blood collection site for liquid biopsy? How do CTCs relate to the primary tumor? Which CTCs matter the most, and how do we identify them? Even if we can genetically characterize the most malignant CTCs, how do we make the most of those data for diagnosis and treatment?
The full clinical potential of CTCs is not yet evident but should become clearer in the next few years. What we ultimately hope to achieve is personalized medicine for cancer diagnosis, prognosis, and treatment monitoring, so we can administer the right treatment regimen to the right patient at the right time.
References
1. A. F. Chambers, A. C. Groom, I. C. MacDonald, Nat. Rev. Cancer 2, 563 (2002). https://doi.org/10.1038/nrc865
2. T. R. Ashworth, Aust. Med. J. 14, 146 (1869).
3. P. Mehlen, A. Puisieux, Nat. Rev. Cancer 6, 449 (2006). https://doi.org/10.1038/nrc1886
4. E. Pailler et al., J. Clin. Oncol. 31, 2273 (2013). https://doi.org/10.1200/JCO.2012.44.5932
5. K. Koyanagi et al., Clin. Cancer Res. 14, 7391 (2008); https://doi.org/10.1158/1078-0432.CCR-08-0290
S. Hoshimoto et al., J. Clin. Oncol. 30, 3819 (2012). https://doi.org/10.1200/JCO.2011.40.08876. M. Wendel et al., Phys. Biol. 9, 016005 (2012). https://doi.org/10.1088/1478-3967/9/1/016005
7. M. Cristofanilli et al., New Engl. J. Med. 351, 781 (2004). https://doi.org/10.1056/NEJMoa040766
8. M. Giuliano et al., Breast Cancer Res. 13, R67 (2011). https://doi.org/10.1186/bcr2907
9. B. Franken et al., Breast Cancer Res. 14, R133 (2012). https://doi.org/10.1186/bcr3333
10. V. Plaks, C. D. Koopman, Z Werb, Science 341, 1186 (2013). https://doi.org/10.1126/science.1235226
11. P. R. C. Gascoyne et al., Electrophoresis 30, 1388 (2009). https://doi.org/10.1002/elps.200800373
12. G. Vona et al., Am. J. Pathol. 156, 57 (2000). https://doi.org/10.1016/S0002-9440(10)64706-2
13. S. J. Tan et al., Biosen. Bioelectron. 26, 1701 (2010). https://doi.org/10.1016/j.bios.2010.07.054
14. H. W. Hou et al., Sci. Rep. 3, 1259 (2013). https://doi.org/10.1038/srep01259
15. J. Shendure, H. Ji, Nat. Biotechnol. 26, 1135 (2008). https://doi.org/10.1038/nbt1486
16. L. B. Alexandrov et al., Nature 500, 415 (2013). https://doi.org/10.1038/nature12477
17. E. Heitzer et al., Cancer Res. 73, 2965 (2013). https://doi.org/10.1158/0008-5472.CAN-12-4140
18. M. Ignatiadis et al., PLoS One 6, e15624 (2011). https://doi.org/10.1371/journal.pone.0015624
More about the authors
Chwee Teck Lim is a professor of biomedical and mechanical engineering and a principal investigator in the Mechanobiology Institute at the National University of Singapore. Dave Hoon is a professor and the director of molecular oncology at the John Wayne Cancer Institute in Santa Monica, California.




