Atomic hearts: A decade of US government-sponsored development
DOI: 10.1063/PT.3.3168
If there’s a chance, any chance at all, that problems caused by technology could outweigh the benefits, we should stop. Trouble is, I hardly know any scientists who will dare stay, ‘Stop.’
—Dr. William Bradfield in Heartbeat.
1
If there’s a chance, any chance at all, that problems caused by technology could outweigh the benefits, we should stop. Trouble is, I hardly know any scientists who will dare stay, ‘Stop.’
—Dr. William Bradfield in Heartbeat. 1
Published in 1978, Heartbeat is a medical disaster novel by Eugene Dong and Spyros Andreopoulos that foretells the perils of an atomic heart. It is a story of William Bradfield’s daring efforts to save the life of a dying patient through the implantation of a mechanical heart powered by plutonium. His patient, Henry Gray, survives the experimental procedure, makes an impressive recovery, and is discharged from the hospital to resume his life. Both Bradfield and Gray enjoy their newfound celebrity as guest speakers describing their experience with the radioisotope-powered artificial heart, and Bradfield goes on to implant more hearts with similar success. But then Gray is kidnapped by a madman who intends to remove and spray the heart’s hundred grams of Pu into the air, exposing thousands of people to dangerous levels of radiation. The Federal Bureau of Investigation and local police begin a manhunt, while the National Heart Institute, government officials, and emergency-services personnel discuss contingency plans in the event that Pu contaminates the area. A life-saving technology for one person has become a threat to society at large.
Heartbeat is fictional, but the technology it depicts is not. Between 1967 and 1977, medical researchers and engineers in two separate, federally funded US programs tackled the technological complexity of designing a mechanical heart whose primary power comes from the heat generated by radioactive decay. It was an ambitious and controversial undertaking. Project scientists claimed that atomic hearts were feasible and practical, but political and social forces challenged that medical assertion throughout the development. The novel’s speculations reflected public anxiety about the risks associated with atomic power, which significantly contributed to the failed development of such devices.

A nuclear-powered pacemaker was implanted in Beagle Brumhilda in 1969 at the National Heart Institute of the National Institutes of Health, Bethesda, Maryland. (Courtesy of the US Department of Energy.)

Competing programs
Large-scale, federally funded science and technology projects, such as the Apollo program and the Superconducting Super Collider, proliferated during the 1960s and 1970s, bolstered by enthusiastic reports from the scientific community with assertions of future benefits for Americans. One federally funded project was the development of atomic hearts to save the increasing number of Americans dying of heart failure during that period. It was industrial scientists, not academic ones, who first proposed exploring radioisotopes as energy sources for artificial hearts. Their timing was ideal, given the recent establishment of the Artificial Heart Program in 1964, at the National Institutes of Health, which sustained the potential and promise of early yet crude device research of the 1950s and early 1960s and the federal government’s support for the peaceful use of nuclear energy. 2 For researchers, the two largest challenges were to build a mechanical pump that was biocompatible—that is, one that a human body could tolerate and that did not damage red blood cells or induce blood clotting—and to incorporate a mechanism for safely and efficiently driving the pump.
The Thermo Electron Engineering Corp of Boston proposed a radioisotope power source both to the National Heart Institute (NHI), later renamed the National Heart and Lung Institute (NHLI), and to the US Atomic Energy Commission (AEC). The corporation hoped to tap into funding from both agencies. AEC chairman Glenn Seaborg was engaged in developing isotopic power units; the most common of them was the radioisotope thermoelectric generator, which produces electricity from the heat of radioactive decay. Chief of the AEC’s thermal applications branch, William Mott, explained: “We were always on the alert for new problems to match with our solutions.” 3 And the radioisotope thermoelectric generator was a solution looking for a problem as industry sought applications beyond spacecraft and remote navigation beacons.
Although the NHI and AEC expressed interest in pursuing the research, both rejected Thermo’s bid, citing the company’s inability to capture the complexity of artificial-heart systems in its proposal. Neither agency rejected the concept, though. The possibility of building an atomic heart appealed to their respective political aims: The NHI sought to expand its fledgling artificial-heart program and build on the Johnson administration’s interest in heart disease, and the AEC welcomed the project as being contiguous to its work on radioisotope-powered space and medical applications. 4
A governmental committee on atomic energy instructed the two agencies to negotiate an integrated, interagency plan to develop an atomic heart, but the NHI and AEC failed to do so. The failure led to two federally funded but independent programs, each pursuing a different approach to design and testing. The NHI proposed developing a short-term-assist heart device, or partial artificial heart, in two stages: A non-radioisotope-powered pump system would come first, followed by a radioisotope-powered engine. The AEC argued that integrating an engine so powered into a mechanical pump not specifically designed for it would not be straightforward. It planned instead to develop an integrated pump and engine as an implantable system that would completely replace the diseased heart on a long-term basis—a loftier, more expensive goal.
The AEC atomic heart
After a competitive bidding process, the Westinghouse Electric Co received a contract from the AEC in 1971 to develop their proposed radioisotope-powered heart system. The agency had decided that Westinghouse’s Stirling engine—which operated by the cyclic compression and expansion of air at different temperatures, powered by a radioisotope thermoelectric generator—was the better-understood and better-developed option for converting heat to work. It was also the most efficient in the desirable size range, was potentially more reliable because of its need for few seals and bearings, and required the least power. The envisioned prototype consisted of two main subsystems—a heat converter and a pump, as shown in figure 1. The work of fabricating the AEC–Westinghouse heart required the expertise of both mechanical engineers and medical scientists, and Westinghouse subcontracted the construction of the heat converter to the engineering firm of Philips of North America, the leading expert in the Stirling engine.

Figure 1. A nuclear-powered artificial heart, designed in the early 1970s by the Atomic Energy Commission for long-term use but never implanted into any test animal. The atomic heart comprises a heat converter and blood pump, both intended to permanently replace the diseased (and removed) human heart. The heat converter was a gas-driven Stirling-cycle engine powered by 60 grams of plutonium-238 encapsulated in metal alloy for safety and thermally insulated to prevent tissue damage. The plutonium heated air, whose expansion pushed a piston, which turned a flywheel and drove the blood pump via a flexible shaft. The pump, a two-ventricle device designed to draw oxygenated blood from the lungs and expel it to the body, operated by the compression of the diaphragms on pusher plates attached to a Scotch yoke—a mechanism that converts a rotating drive shaft into the linear motion of a slider. Lining the components with rubbery silicone plastic, thinly textured with Dacron polyester fibrils, helped reduce blood clotting and the risk of an embolism or stroke. (Courtesy of Special Collections, J. Willard Marriott Library, University of Utah.)
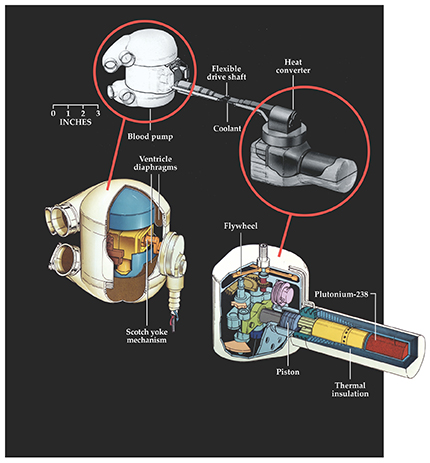
The heat converter produced by Philips was a gas-driven machine, powered by 33 W extracted from 60 grams of plutonium-238. After considering such radioisotopes as promethium-147 and thulium-171, Philips chose 238Pu because of its low emission rate, high power density, long half-life (87.7 years), and availability. Recognizing the toxicity of 238Pu, Philips’s engineers triply encapsulated the material in high-strength alloys of platinum, rhodium, and tantalum for safety and durability. They also thermally insulated the engine to reduce heat dissipation, and hence tissue damage, in the body.
To assemble the blood pump, Westinghouse worked with Willem Kolff, shown in figure 2, and his research colleagues at the University of Utah. Westinghouse’s astronuclear lab built the mechanical pump parts and collaborated with Kolff’s team on the pump’s design and biomaterials fabrication. Together they also constructed the flexible drive shaft connecting the pump to the heat converter. The pump consisted of two ventricles that circulated blood by the compression of two diaphragms on pusher plates. Through a gearing system and yoke, the 1800 revolutions per minute output of the Stirling engine was reduced to actuate the pump diaphragms at 120 beats per minute.

Figure 2. Willem Kolff (1911–2009). Inventor of the artificial kidney and a pioneer in artificial-heart development,

The plan was to orthopedically fit the entire system into the body of a patient: Surgeons would squeeze the pump into a chest whose diseased biological heart had been removed and then connect it through the flexible drive shaft to the heat converter implanted in the abdomen. 5 By coating the pump’s rubbery silicone plastic ventricles with Dacron polyester fibrils, to which blood platelets clumped and produced a smooth yet thin cellular layer, researchers hoped to reduce blood clotting in the pump. But by 1972 the AEC–Westinghouse heart remained far from ideal: Both heat converter and blood pump were too big, heavy, inefficient, and unable to meet the body’s energy needs—the so-called load profile. Nevertheless, Westinghouse officials were encouraged by the progress and committed the next several years to improved fabrication and testing, with animal implants scheduled for 1974.
Their optimism and confidence, however, were exceeded by those of NHLI officials, who beat their rivals to the punch. The first animal implantation of a nuclear-powered artificial heart system happened not with a complete AEC–Westinghouse system but with an NHLI device that assisted an ailing but still beating heart.
The NHLI atomic heart
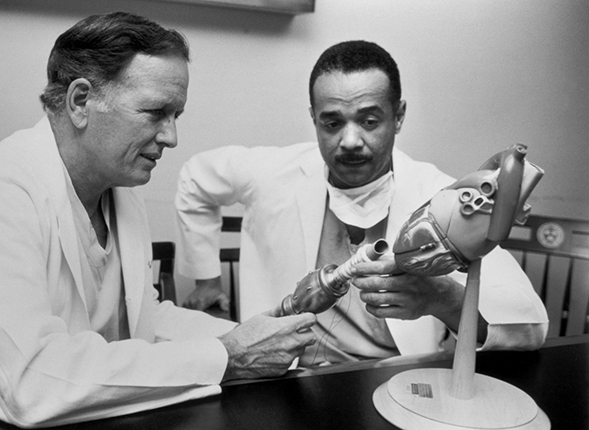
In February 1972 cardiac surgeon John Norman Jr, shown in figure 3, implanted a ventricular assist pump into a calf. Powered by 238Pu, the pump operated for eight hours until a kinked inflow tube ended the experiment. The NHLI’s director issued a press release to announce the achievement, and the story was front-page news nationwide.

Figure 3. Denton A. Cooley and John C. Norman Jr discuss the placement of a left ventricular assist device, a partial artificial-heart system designed to temporarily sustain patients with failing hearts. Between 1972 and 1974, Norman (right) tested early versions of his cardiac-assist device, powered by plutonium-238, in 15 calves in the research laboratories at Harvard University’s School of Medicine and the Texas Heart Institute in Houston, but with poor results. The calves survived for hours, not days, and problems included device leaks or breaks and internal heat injuries. Norman continued to develop improved pumps with Cooley at the Texas Heart Institute but abandoned 238Pu as a power source. (Courtesy of the Texas Heart Institute.)
Like the AEC–Westinghouse device, the NHLI’s assist system consisted of two main parts: a heat converter and a blood pump, arranged in the calf’s body as shown in figure 4. The pump attached to the left ventricular apex of the heart and to the descending thoracic aorta, a configuration that allowed the pump to assist the movement of oxygenated blood. Also like the AEC–Westinghouse device, the pump was made of Dacron-fibril-covered silicone and plastic, and it forced blood through the pump’s bladder using a pusher plate. The bladder was clamped in stainless-steel housing and hydraulically driven by an attached 3 kg cylinder, situated in the abdomen, that contained the nuclear heat converter.

Figure 4. A nuclear-powered heart-assist device, sponsored by the National Heart and Lung Institute (NHLI) for short-term use, was tested in more than a dozen calves. This sketch, published in 1972, illustrates its main parts—a ventricular assist pump and a plutonium-powered steam engine. The pump was attached to a failing heart to assist the circulation of oxygenated blood from the lungs to the rest of the body. More specifically, the pump accepted blood from the left ventricle and ejected it into the descending thoracic aorta. Connected to the pump via hydraulic lines, a 3 kg cylinder wrapped in Dacron bands and containing the 0.7 kg engine (the pneumatic-hydraulic converter) and 120 g of 238Pu was implanted in the abdomen. The engine, whose operating temperature was about 480 °C, converted the radioisotope’s heat into 52 W of hydraulic power. (Adapted from ref.
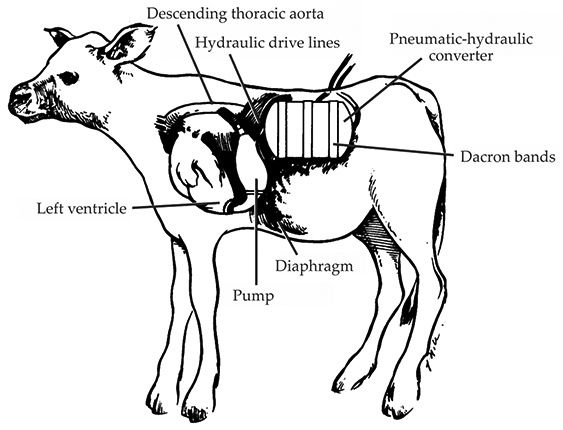
Developed by Thermo, the NHLI’s heat converter differed from Philips’s in its mechanics and needed twice the amount of 238Pu: About 120 g generated 52 W of hydraulic power to drive the pump. Unlike a Stirling engine, in which the working gas (air) never changes phase, Thermo’s so-called tidal-regenerator engine vaporized and condensed a water droplet during each expansion and compression cycle. The familiar steam engine is an example of such a mechanism. Advocates of the engine argued that the system’s fewer moving parts—it contained no valves or sliding seals—made it preferable over other nuclear engines under development.
The NHLI took advantage of the timing of Norman’s animal implants to release a 63-page report outlining the team’s “substantial progress” in nuclear-engine development and blood-pump systems from five years of NHLI-funded research. 6 To judge from its simplified presentation and confident tone, the report was most likely meant to reassure senior management and public officials. Written for lay rather than scientific consumption, it was dismissed by many in the field as a political document. Many of the technological gains declared as NHLI successes were hardly unique to the NHLI’s nuclear-powered heart program.
Researchers at the AEC and other critics flatly accused the NHLI of overstating its results. No NHLI atomic heart—nor any such device—was nearing clinical use. One anonymous critic (possibly Mott) denounced the NHLI statement as “full of deceit” and delivered for the purpose of obtaining funding from Congress. 7 AEC researchers warned Congress not to be misled because the NHLI engine technology showed no major advancement since it had last been reviewed in June 1970. According to Mott, the NHLI report was “the greatest piece of technology charlatanism that has come down the pike in a long time.” 8 In response to all that criticism, the NHLI released another statement conceding the technical bugs in its system, admitting to engine overheating and blood clotting in the pump.
After only four animal implants in early 1972, Norman stopped his testing, pending improvements to the system. In 1973 and 1974, he implanted another 11 calves, but his own comments and the obvious technical problems suggested that the earlier NHLI success statements were premature. Indeed, the exaggerated claims only made the public more suspicious at a time when media reports, lawsuits, and vocal public outrage abounded concerning reported deaths and injuries from defective heart valves, pacemakers, and other medical devices.
Should atomic hearts be built?
Even if experts resolve the technological problems, are nuclear-powered hearts desirable? Are the risks associated with radioisotopes acceptable? And who should judge? Experts, government officials, and bioethicists began to ask such questions in the wake of debates on the safety of medical devices at the time. According to Mott, “Without question a plutonium-238 powered heart, regardless of its technological assets, will stir many more emotions and evoke much stronger criticism than would a heart powered by any other means.” 3
The development of radioisotope-powered pacemakers raised the same kind of issues. Fuel cells, chemical batteries, and betavoltaic power sources, which used less-energetic beta-emitting radioisotopes, were also under development but gained little traction with heart-pump researchers. (See the article by Larry Olsen, Peter Cabauy, and Bret Elkind, Physics Today, December 2012, page 35
The NHLI attempted to get in front of the debate by convening a mixed medical and lay panel to examine the broader social, ethical, legal, and economic implications of the development and use of artificial hearts in humans. The panel submitted a 250-page report recommending that research on all types of mechanical circulatory support systems continue with NHLI funding. Moreover, it supported a nuclear-powered approach as the most promising technological option for a mechanical heart. At the time, biological fuel cells were decades away from being practical, and battery systems, which tended to overheat, had a two-year life span and required daily recharging.
Yet the panel was uneasy about the toxicity of Pu, claims by AEC and NHLI scientists that the fuel capsule was indestructible, and the possibility of accidents or criminal acts of the sort later dramatized in Heartbeat. Little was known about the biological effects of continuous exposure to low-dose radiation, and the National Council on Radiation Protection and Measurements argued that the estimated exposure surrounding a Pu implant would put an atomic heart recipient at risk of sterilization and leukemia, among other health problems. By extension, caregivers and family members were also at risk of radiation poisoning, as was the general public, which may unknowingly be exposed to the recipient. 10
The panel thus recommended in 1973 that radioisotope-powered artificial hearts not be implanted in humans until it was established that they impart no significant involuntary risk to others. 11 Kolff and other researchers contested the recommendation and argued that human tests could supply data impossible to get from studies on other animals. The panel was not persuaded and pointed to the danger of a slippery slope: The widespread use of atomic systems might not be controllable once human implants, experimental or otherwise, began.
The NHLI responded immediately to almost all of the panel’s specific recommendations by discontinuing support for its atomic heart program and redirecting its attention to other energy sources. Unsatisfactory animal tests of three different agency-sponsored heat engines with various ventricular assist devices produced a discouraging outlook for nuclear-powered devices. And the incumbent AEC chairman, Dixy Lee Ray, announced that the AEC would phase out the development of its atomic heart over the next three years. A 1974 review of the AEC’s atomic heart program by a group of seven independent engineers and research physicians criticized its device as “immensely complicated with more than a dozen gears and heaven knows how many bellows and bearings. It is difficult for most to conceive of such a device working successfully for ten years without service.” 12
Contributing to the pessimism was the budget crunch of the mid 1970s. Many government officials deemed the atomic heart program too long-term and costly to continue, and drastic budget cuts seemed imminent. By 1977 institutional support for atomic heart programs had ended.
Alternative energy
Despite scientists’ assertions that the technological complexity of the atomic heart was surmountable, public concern contributed to the government’s decision to withdraw funding. Several factors eroded the public’s confidence in the scientists’ claims. Competition between the two governmental agencies probably hindered the development of a functioning atomic heart, and the sniping between them may have led to skepticism of stated progress. What’s more, the scientific community was not unified in its support for either the development of an atomic heart or the role of outsiders in assessing the research programs. The programs’ decade-long duration is testimony to the commitment of a handful of researchers.
Work on artificial hearts went on, albeit without a nuclear power source. Kolff’s lab, for instance, returned to an older pneumatic source, and later an electric one, to drive its pumps. Kolff’s best known device, the Jarvik total artificial heart, was implanted experimentally in human patients during the 1980s, first as a permanent device and later as a bridge until a donor heart became available. But device problems and ambiguous patient outcomes drew medical and public criticism; in 1990 the Food and Drug Administration withdrew approval for its experimental use, citing company record-keeping deficiencies.
The technology reemerged as the SynCardia total artificial heart, and in 2004 it became the first complete artificial heart to secure FDA approval for commercial marketing. Left ventricular assist devices (LVADs) have enjoyed greater commercial and clinical success, starting with experimental patient implantations in the 1980s followed by FDA approval for the pneumatically driven, pulsating HeartMate LVAD in 1994. Former vice president Dick Cheney lived with a second-generation, continuous-flow HeartMate pump for 20 months in 2010–12 before receiving a heart transplant. This year at least another 2600 Americans in advanced heart failure will receive an LVAD, based on a North American device registry (www.uab.edu/medicine/intermacs
This article is based on McKellar’s article “Negotiating risk: The failed development of atomic hearts in America, 1967–1977,” Technology and Culture
References
1. E. Dong, S. Andreopoulos, Heartbeat, Coward, McCann, & Geoghegan (1978).
2. A. N. H. Creager, Life Atomic: A History of Radioisotopes in Science and Medicine, U. Chicago Press (2013);
A. Kraft, Contemp. Br. Hist. 20, 1 (2006). https://doi.org/10.1080/136194605004449403. W. E. Mott lecture, “Nuclear Power for the Artificial Heart” (17 October 1973), folder 3, box 168, MS 654, Willem J. Kolff Collection, Special Collections, J. Willard Marriott Library, University of Utah, Salt Lake City.
4. V. A. Harden, Inventing the NIH: Federal Biomedical Research Policy, 1887-1937, Johns Hopkins U. Press (1986);
A. L. Buck, A History of the Atomic Energy Commission, US Department of Energy (1983).5. L. Smith et al., Trans. Am. Soc. Artif. Intern. Organs J. 20, 732 (1974).
6. L. T. Harmison, “Totally Implantable Nuclear Heart Assist and Artificial Heart” (February 1972), box 1, MS C 578, Acc. 2003-054, John T. Watson Papers, Modern Manuscripts Collection, History of Medicine Division, National Library of Medicine, Bethesda, MD.
7. Artificial Hearts News Release (6 October 1972), folder 10, box 300, MS 654, Kolff collection, in ref. 3.
8. W. E. Mott, comments on NHLI announcements of March 2, 1972 (15 March 1972), folder 23, box 7, MS C 574, Clarence Dennis Papers, Modern Manuscripts Collection, History of Medicine Division, National Library of Medicine, Bethesda, MD.
9. For more on atomic pacemakers, see V. Parsonnet et al., Pacing Clin. Electrophysiol. 29, 195 (2006). https://doi.org/10.1111/j.1540-8159.2006.00317.x
10. National Council on Radiation Protection and Measurements, Basic Radiation Protection Criteria, report no. 39, NCRP (1971);
Helen Caldicott, Nuclear Power Is Not the Answer, New Press (2006).11. National Heart and Lung Institute, Artificial Heart Assessment Panel, The Totally Implantable Artificial Heart: Economic, Ethical, Legal, Medical, Psychiatric, Social Implications, National Institutes of Health (1973).
12. C. Dennis to W. E. Mott (7 June 1974), folder 10, box 6, MS C 574, Dennis papers, in ref. 8.
13. S. McKellar, in Essays in Honour of Michael Bliss: Figuring the Social, E. A. Heaman, A. Li, S. McKellar, eds., U. Toronto Press (2008), p. 400.
14. J. C. Norman et al., Ann. Surg. 176, 492 (1972). https://doi.org/10.1097/00000658-197210000-00007
More about the authors
Shelley McKellar is the Jason A. Hannah Chair in the History of Medicine at Western University in London, Canada. She is writing a book on the history of artificial hearts.





