An atomic physics perspective on the kilogram’s new definition
DOI: 10.1063/PT.3.4472
Although often the bane of freshman physics students, units of measure are important for applications from commerce to fundamental physics. The current Système International (SI) units emerged early in the French Revolution to unify and promote égalité (“equality”) in commerce. Over the past two centuries, major changes and updates to SI units have occurred, but the redefinitions introduced on 20 May 2019 were the biggest conceptual transformation in metrology since the French Revolution.

The platinum–iridium cylinders are replicas of the one in Paris that defined the kilogram until 2019. (Courtesy of J. L. Lee/NIST.)

All prototype-based definitions have now been replaced with ones based on the cesium atom and fundamental constants. (See the article by David Newell, Physics Today, July 2014, page 35
From artifacts to fundamental constants
Over time, physicists have defined and redefined units based on natural objects, then objects of human creation and scale, and finally microscopic objects paired with fundamental constants. For example, the meter was first defined as one ten-millionth of the distance from the equator to the North Pole. In 1799 that standard was replaced by a manufactured prototype meter bar, which was more precise. But the accuracy was still limited to 10−7, and calibration measurements required a precise temperature and pressure; also required was that the meter bar be supported at the so-called Airy points, for which bending is minimized.
In 1960 the CGPM introduced a microscopic reference by defining the meter as a specific number of wavelengths of the emission from a transition in krypton-86. Now every laboratory in the world could create their own standard. But the length standard was still tied to a specific atomic transition, and with the development of lasers, krypton was no longer the best choice available. Instead of picking another atomic or molecular line, in 1983 CGPM defined the speed of light as 299 792 458 m/s and the meter as the distance travelled by light in vacuum in 1⁄299 792 458 of a second. In practice, because researchers can measure time and frequency much more accurately than length, they measure any laser’s frequency and then convert it to a wavelength using the speed of light. With a defined
The kilogram’s definition has a history similar to that of the meter. Originally, in 1795 the kilogram was defined as the mass of one liter of pure water at the melting point of ice. But unavoidable impurities in the water limited the precision. Manufactured prototypes were thus developed. In 1799 a cylinder made of platinum was introduced, and in 1879 one made of platinum–iridium, shown in the opening image on page 32, came into use; it provided the definition of the kilogram for 140 years. The problem was that when the original platinum–iridium cylinder, or the urkilogram, and its copies were compared after a year, their masses differed by up to 50 micrograms, likely because atoms fell off or hydrogen was absorbed from air contaminants or cleaning products.
The urkilogram was difficult to replace because microscopic and macroscopic masses differ by a factor of 1025, which is hard to measure accurately; in contrast, the meter is on the order of 106 optical wavelengths. To define the kilogram in a microscopic way, metrologists could have used the mass of a specific number of a specific atom, or of electrons or protons, similar to the meter’s definition in terms of krypton radiation. Instead, the new definition relied on a set value for a fundamental constant, similar to the meter’s definition through the speed of light. For the kilogram, the fundamental constant was Planck’s quantum
With the new definition of the kilogram, all unit definitions in physics rely on microscopic quantities or fundamental constants and no longer involve manufactured artifacts. Any laboratory in the world can create primary standards; Paris, the home of the meter bar and the urkilogram, has lost its special role.
Kilogram in terms of frequency
Time is central to the SI because it can be measured in terms of frequencies much more precisely than any other quantity. The International Committee of Weights and Measures defines the second as “the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium-133 atom”; that is, the Cs hyperfine frequency is defined as 9.192 631 770 GHz. Cesium is a practical choice: Its frequency is convenient, and its heavy mass leads to low atomic velocities and thus to small transit-time spectral broadening. A single photon at the Cs hyperfine frequency
Because total energy is conserved, if a Cs atom absorbs a photon of frequency
In the new definition, one kilogram is the mass difference between
Figure 1.

The new kilogram definition can be understood as (a) the mass difference between 1.475 521 4 × 1040 cesium atoms in the ground state and the same number in the excited hyperfine state or as (b) the mass of 1.475 521 4 × 1040 photons at the Cs hyperfine frequency trapped in a microwave cavity. The definitions of Planck’s constant
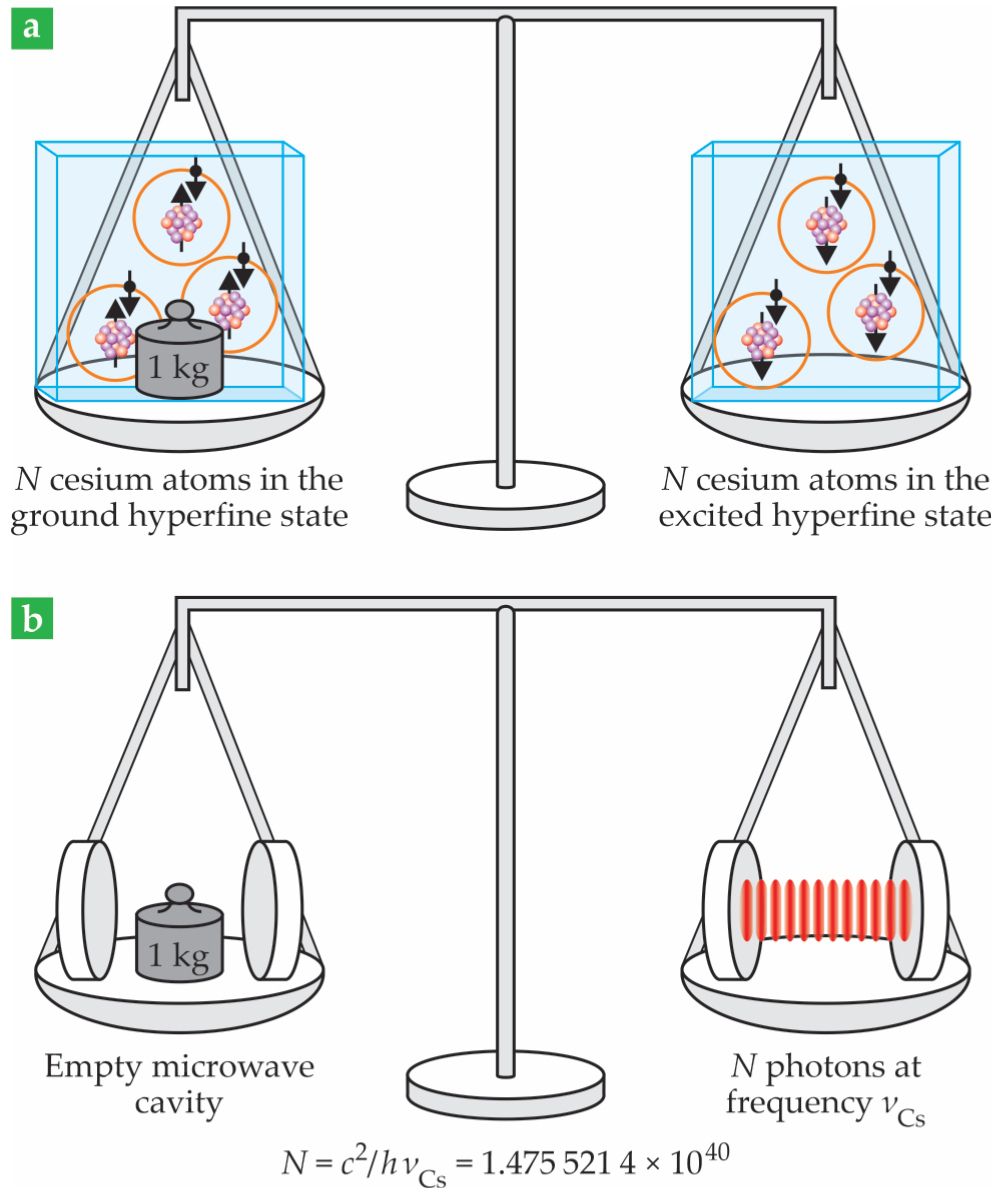
A Cs atom isn’t even necessary to turn a photon’s relativistic mass into rest, or invariant, mass. If photons are in a cavity, their total momentum is zero, and they are at rest in the lab reference frame. The composite rest mass is the mass of the empty cavity plus the photons’ relativistic masses. The kilogram can then be defined as the mass of 1.475 521 4 × 1040 photons at
The definition above is impractical or even impossible for calibrating masses. But through highly accurate (10−20) frequency ratios and frequency combs, researchers can use any radiating system to define the kilogram. As a simple but still impractical example, they could annihilate an electron and a positron to create radiation at a frequency linked directly to the electron mass
Kilogram through atomic spectroscopy
A kilogram based on gamma rays is not practical, given how difficult it is to measure their frequencies with high precision. So instead of converting the rest energy
In hydrogen spectroscopy, the electron has a well-known velocity. Besides some small well-understood corrections, the ionization energy of hydrogen is the Rydberg energy
Hydrogen spectroscopy is thus a feasible way to measure the mass of the electron. The experimental realization has a precision of 2 × 10−12 for the Rydberg constant, limited by the uncertainty in the finite size of the proton.
5
But how does the mass of the electron define a macroscopic mass? The first step is to relate the mass of the electron to the masses of atoms. An electron and an atomic ion placed sequentially in a Penning trap have cyclotron frequencies
The International Avogadro Coordination (IAC) project, led by Physikalisch-Technische Bundesanstalt (PTB) in Germany, took the remaining step to convert microscopic to macroscopic mass. The project members used hydrogen spectroscopy and Penning trap mass comparisons to connect
Figure 2.

A silicon sphere like the ones used by the International Avogadro Coordination. The IAC measured the diameters and lattice constants of the nearly perfect spheres to count the atoms and convert from the mass of a silicon atom to the mass of the silicon sphere. (Courtesy of NIST.)

Atom interferometry
Hydrogen spectroscopy connects the mass of the electron to the hyperfine frequency of cesium by measuring the electron’s kinetic energy in the ground state. For those measurements, small corrections from quantum electrodynamics effects and the finite proton size must be determined by other measurements. Atom interferometry, on the other hand, directly determines the kinetic energy of an atom by a frequency measurement.
In an atomic recoil measurement, a photon transfers to an atom a precise momentum,

A pulse of counterpropagating laser beams (green arrows traveling along the dashed green lines in panel a) at different frequencies places an atom, initially at rest (solid black line) into a superposition of moving and resting states (solid gray lines). A second laser pulse with both beams at the same frequency reverses the direction of the moving arm without disturbing the resting arm. A final pulse, identical to the initial pulse, reads out the arms’ phase difference, which results from the kinetic energy of the moving arm, through the relative populations in the two output ports of the interferometer (dashed black lines).
When an atom scatters a photon from one beam into the other after each pulse, as shown in panel b, it receives a momentum transfer
After the momentum transfer, an atom initially at rest has a kinetic energy
The IAC researchers started by creating the world’s most perfect sphere out of single-crystal isotopically enriched silicon. They determined the sphere’s diameter
The conceptual definition of the kilogram involved 1.475 521 4 × 1040 photons at the Cs hyperfine frequency. Instead of counting 1040 photons, the Avogadro project counted approximately 1025 silicon atoms. The mass of a silicon atom is 15 orders of magnitude larger than the relativistic mass of a Cs photon. Those additional 15 orders of magnitude come in roughly equal factors from comparing frequencies
Kibble balance
A final method to realize a mass standard is the Kibble balance, formerly called the Watt balance. Until 2019 the Kibble balance was used to measure the value of Planck’s constant
Kilogram with the Kibble balance
In the main text, the concept of the Kibble balance is explained through energy conservation: The electrical power required to levitate an object of mass

In force mode (panel a in the figure), the Kibble balance magnetically levitates the test object. The Lorentz force
In velocity mode (panel b), the Kibble balance moves the solenoid at a constant velocity
With the current measured in force mode and the voltage measured in velocity mode, their product
The basic idea is to measure a change in mechanical energy, which is proportional to the mass of an object, using electrical power. When a motor lifts an object with velocity
In the Kibble balance, the motor is replaced by a levitating coil with current
Researchers use the Josephson effect to measure the product of voltage and electron charge,
Single-electron pumps operate with currents up to 100 pA at frequencies
8
near 1 GHz and count the macroscopic number of electrons per time,
With electrical power measured that way, the Kibble balance realizes masses by obtaining the energy, or voltage times
The accuracy of current standards based on single-electron-pumps
8
is around 10−7, so researchers chose a different way to measure the current. It passed through a quantum Hall device kept at a resistance plateau equal to the von Klitzing constant
The new definition of the kilogram has the advantage not only that it can be realized everywhere in the world, independent of artifacts, but also that it can be directly applied to objects at any mass. For a 1 kg object, the precision of the Kibble balance and silicon spheres is about the same as the urkilogram. But for smaller masses, the old definition required dividing masses using pairwise comparisons of equal masses, and each order of magnitude typically involved five weights. 2 Reaching milligrams, for example, required many steps with huge losses in precision, whereas the new definition works the same for a kilogram or a milligram.
The various routes to the new kilogram can be reduced to connecting a frequency to the mechanical or relativistic energy of a particle. Conceptually, that means using the relativistic energy of a particle (through annihilation radiation). Practically, it means using the kinetic energy of a microscopic object with either a well-known velocity (electron in the hydrogen atom) or momentum (atom interferometry) or of a macroscopic object with well-known velocity and acceleration (Kibble balance).
Do you understand the new definition of mass? Take the quiz:
1. In the new SI units, is the mass of one mole of carbon exactly 12 g or does it now have an experimental uncertainty?
2. In the new SI units, does the weight of a 1 kg object have an accuracy better than 10 µg, the limit for comparing the urkilogram to its copies?
3. Do the new SI units reduce the uncertainty of microscopic masses, such as the mass of the electron?
4. Do the new SI units increase the uncertainty of the mass of certain objects?
5. Would it be possible to define the units of time, length, and mass without referring to any natural or artificial particle—for example, an electron, hydrogen atom, photon at the cesium hyperfine frequency, or urkilogram—by fixing the numerical values of fundamental constants, or is at least one such particle always needed?
(For answers see
Planck units
The new SI units almost reach Max Planck’s 1899 vision to define all units using only fundamental constants without reference to specific particles.
9
Planck suggested defining the gravitational constant
First, what is the Planck mass? If the mass of a point-like object increases, its reduced Compton wavelength
To measure masses in units of the Planck mass, a gravitational effect must be measured at some distance
A more elegant but equally unrealistic method is the gravitational redshift
Unlike Planck’s vision, the new SI units still use one specific particle, the cesium atom, although that unique role will soon likely pass to another atom with an optical frequency, such as strontium, ytterbium, ytterbium ions, or aluminum ions. 10 Planck argued that the selection of an atom and a spectral line to define frequency is arbitrary, whereas definitions based on fundamental constants would be valid for all times and all cultures, including extraterrestrial and extrahuman cultures (“ausserirdische und aussermenschliche Culturen”). 9
Planck’s units and the new SI units could, in principle, be fundamentally different. For example, if the expectation value of the Higgs field slowly changes as a function of position, the masses of elementary particles would subtly alter, but fundamental interactions would remain unchanged. SI units based on a selected particle would then drift in value from place to place, whereas the Planck units would remain fixed. That example illustrates one of the many connections between metrology and fundamental science.
Did you pass the quiz? Find out here:
1. No, it has uncertainty. Because Avogadro’s number now has a fixed value, the mass of one mole of carbon is no longer exactly 12 g. It has an uncertainty of 3 × 10−10 due to uncertainty in the carbon atom’s mass.
2. No. The experimental uncertainties in measuring the mass of a 1 kg object with the old and new definitions are comparable at 10−8.
3. Yes. The uncertainty in the electron’s mass in kilograms has dropped from 10−8, based on comparisons to the urkilogram, to 3 × 10−10 in the new definition.
4. Yes. The urkilogram in Paris is no longer exactly 1 kg but has an uncertainty of about 10 µg.
5. Yes, it is possible. Planck units, discussed in the last section of the article, rely only on fundamental constants, not on a specific particle.
References
1. D. B. Newell et al., Metrologia 55, L13 (2018). https://doi.org/10.1088/1681-7575/aa950a
2. S. Schlamminger, Redefining the Kilogram and Other SI Units, IOP Publishing (2018), chap. 1.
3. S. Rainville et al., Nature 438, 1096 (2005). https://doi.org/10.1038/4381096a
4. B. N. Taylor, P. J. Mohr, Metrologia 36, 63 (1999). https://doi.org/10.1088/0026-1394/36/1/11
5. A. Beyer et al., Science 358, 79 (2017). https://doi.org/10.1126/science.aah6677
6. S. Sturm et al., Nature 506, 467 (2014). https://doi.org/10.1038/nature13026
7. G. Bartl et al., Metrologia 54, 693 (2017). https://doi.org/10.1088/1681-7575/aa7820
8. F. Stein et al., Appl. Phys. Lett. 107, 103501 (2015). https://doi.org/10.1063/1.4930142
9. M. Planck, Ann. Phys. (Leipzig) 306, 69 (1900). https://doi.org/10.1002/andp.19003060105
10. A. D. Ludlow et al., Rev. Mod. Phys. 87, 637 (2015). https://doi.org/10.1103/RevModPhys.87.637
11. P. Cladé et al., Metrologia 53, A75 (2016). https://doi.org/10.1088/0026-1394/53/5/A75
12. R. H. Parker et al., Science 360, 191 (2018). https://doi.org/10.1126/science.aap7706
More about the authors
Wolfgang Ketterle is the John D. MacArthur Professor of Physics at the Massachusetts Institute of Technology in Cambridge, director of the MIT–Harvard Center for Ultracold Atoms, and associate director of the Research Laboratory of Electronics. Alan Jamison is an assistant professor of physics and a member of the Institute for Quantum Computing at the University of Waterloo in Ontario, Canada.






