Your wetting day
DOI: 10.1063/1.2711652
It’s time to wake up. You pour water to make coffee, then take a shower, but you have difficulty grabbing the bar of soap, which adheres to its holder. After the shower, you comb your wet hair. In the kitchen, you observe condensed water drops inside your refrigerator, cook breakfast in a Teflon-coated pan, and look out a window blurred with water drops that condensed during the night. You walk outside and see drops shimmering on the grass, flowers, and a spider web. At a touch, the drops roll rapidly off the flowers. At work you paint. The bristles of your paintbrush coalesce as you remove it from the paint. You decide to write about what you’ve observed, but the only paper you have does not retain the ink from your pen.
All these phenomena—some at home, some in nature, some in industry—involve wetting, the contact that liquids make with surfaces. To understand wetting, two concepts are essential: the surface tension, also called interfacial tension, which characterizes the energy of the boundary between distinct material phases, and the contact angle, which characterizes the degree to which a liquid is attracted to a substrate.
Surface energy
Interfaces between fluid phases can be viewed as deformable membranes described by a surface tension γ that opposes small deformations from stable equilibrium. Surface tension is a macroscopic parameter that represents the consequences of molecular effects: Molecules at interfaces lose roughly half of the cohesive interactions they would have with their neighbors in the bulk material, and a liquid adapts its shape to minimize its surface energy. You see that effect whenever you blow a soap bubble; the film of liquid minimizes its surface area by adopting a spherical shape. Physically, γ represents the energy required to increase the area of the surface by one unit, and so has units of J/m2. If you’ve ever pulled on a soap film, you may recognize that γ is also a tensile force per unit length acting tangent to the interface.
Since γ characterizes the interface separating distinct fluid phases, one can estimate γ as the cohesion energy divided by the area occupied by a molecule. Taking the cohesion energy to be the thermal energy k B T (k B is Boltzmann’s constant) and a typical molecular length of about 0.3 nm, one finds a room-temperature γ of order 4 × 10−2 J/m2.
Due to surface tension, a pressure jump Δp exists across a curved surface. From dimensional considerations, the pressure difference needed to curve a spherical interface with surface tension γ to a radius R is roughly Δp ≈ γ/R. The exact result, given by Pierre Simon Laplace in 1806 and now called the Laplace pressure, is Δp = 2γ/R. An elementary derivation of Laplace’s result is obtained by considering the work dW to increase the radius of a sphere with surface area S and volume V by a small amount dR: dW = γdS — (Δp)dV. Setting dW = 0, which corresponds to mechanical equilibrium, and using dS/dV = 2/R gives the result. More generally, for any surface, one defines the mean curvature κ as κ = 1/2(1/R 1 + 1/R 2), where R 1 and R 2 are the two principal radii of curvature, and finds Δp = 2γκ. For a sphere, R 1 = R 2 = R, which yields the Laplace pressure.
Contact angle
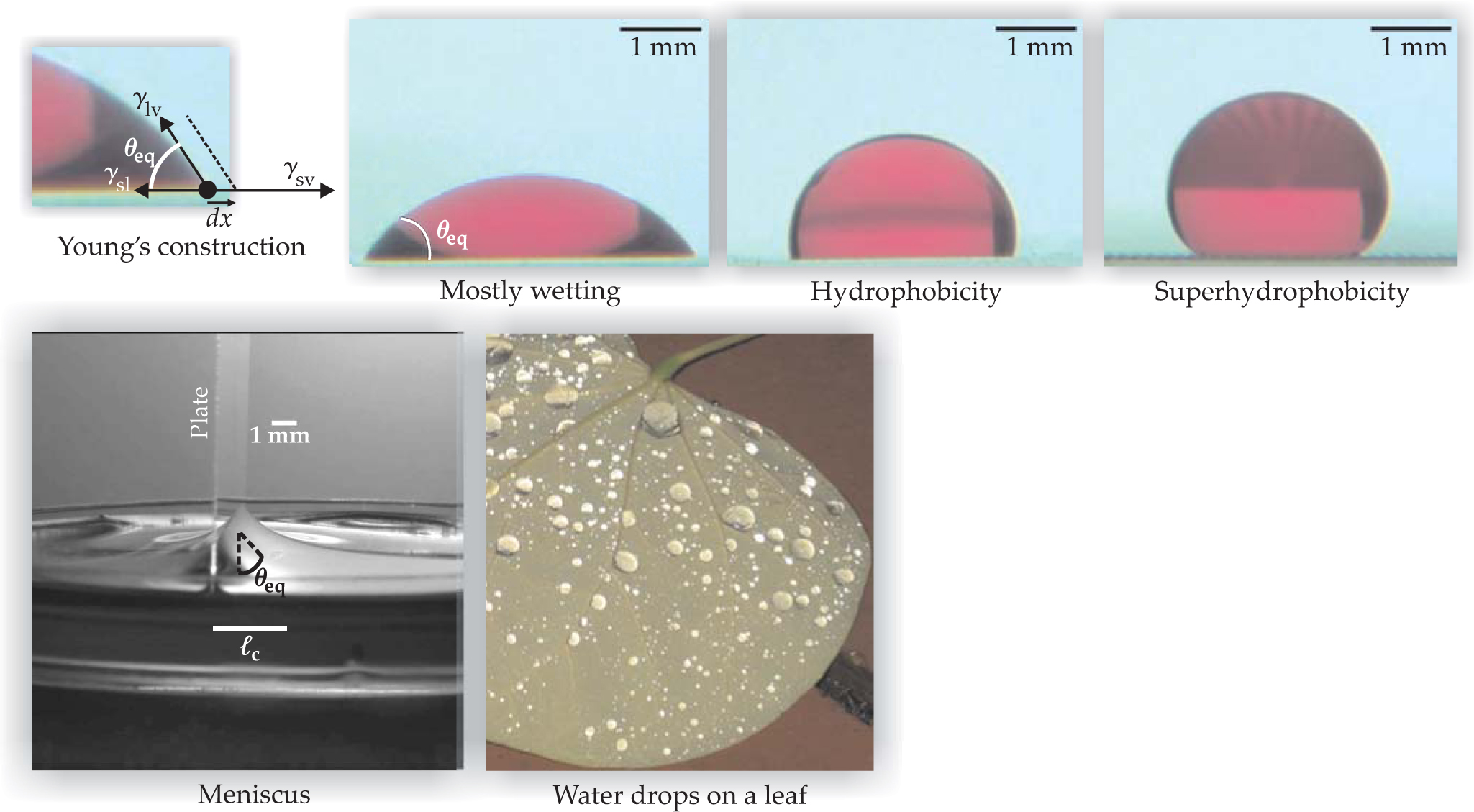
The second key parameter associated with wetting and illustrated on page 85 is the equilibrium contact angle θ eq formed at the “contact line” between the liquid–vapor (l–v) interface and a solid (s) substrate. If γ ijdenotes the surface tension of the interface between phases i and j, then the change in the energy dE per unit length of contact line associated with a small displacement dx along the surface is dE = (γ sl — γ sv)dx + γ lv cosθ eq dx. The minimization corresponding to equilibrium, dE/dx = 0, gives the formula, attributed to Thomas Young in an 1805 paper, that relates the contact angle and the surface tensions: γ cosθ eq = γ sv — γ sl, where γ = γ lv. If 0° < θ eq < 90°, one says the liquid mostly wets the surface. For θ eq ≥ 90° the surface is poorly wet. The ideal case of θ eq = 0° corresponds to perfect wetting.
Changing the substrate affects the right-hand side of Young’s relation; consequently, water droplets placed on different substrates display a variety of wetting behaviors, as illustrated in the figure. A small drop can partially wet a surface and form a spherical cap. Water drops mostly wet hydrophilic surfaces and poorly wet hydrophobic surfaces.
Wetting properties can be changed by chemical treatments, such as coating a surface with a molecular monolayer. Surprisingly, surface roughness can enhance the natural wetting behavior of a substrate. For example, imagine a water droplet on a hydrophobic material with a θ eq > 90° that has been topographically patterned with a roughness scale of approximately 100 µm in both amplitude and wavelength. In this case, as shown in the figure, the surface exhibits superhydrophobicity, and water has an effective equilibrium contact angle of about 150°. A water drop would easily roll on the roughened surface.
Nature exploits both chemical and physical properties of wetting. For example, the lotus leaf combines surface chemistry and roughness at the micron scale to produce superhydrophobicity. In the desert areas of Namibia, the Stenocara beetle uses the superhydrophobicity of its back to capture water from morning fog. After ducks leave water, they dry off rapidly because of the superhydrophobic properties of their feathers. In more exact language, they dewet due to the withdrawal of water from the hydrophobic feather surface via the nucleation of dry spots. Also, the physics behind superhydrophobicity and dewetting is helpful in designing self-cleaning surfaces.

Wetting phenomena. The way a liquid makes contact with a surface is characterized by the equilibrium contact angle θ eq. As described in the text, Young’s construction considers a small displacement dx of the contact line (seen edge-on as a filled circle) and determines the angle. A water drop deposited on different substrates can exhibit various wetting behaviors: It mostly wets a hydrophilic glass plate, poorly wets a hydrophobic flat surface, and displays superhydrophobicity when surface roughness is enhanced, as on some leaves. A meniscus is observed when a glass plate is dipped into water; the deformation vanishes over a length scale
Capillary action
The parameters γ and θ eq are useful for describing the shapes of interfaces and other capillary phenomena, which involve surface forces and other forces such as gravity. For example, a paper clip carefully deposited on the surface of clean water will float, even though the clip is denser than the water. Similarly, water striders may be supported by the surface of a pond. Moreover, as a consequence of Young’s relation, when a liquid is placed in a container, the liquid surface deforms close to vertical walls; the curved region or meniscus, illustrated in the figure, has a typical length scale denoted ℓc. Comparing an estimate of the Laplace pressure, γ/ℓc, to the hydrostatic pressure, ρ gℓc, where ρ is the liquid density and g is the gravitational acceleration, yields the capillary length
The most familiar capillary effect is the rise of mostly wetting fluids confined in narrow tubes. To approximately determine the equilibrium rise H in a tube with radius R, first estimate the change in surface energy for an intermediate height h, E S = 2π Rh(γ sl − γsv), and the change in the gravitational potential energy, E G = ½(π R 2 h 2 ρ g). Equilibrium is established when the variation in total energy d(E S + E G) = 0, which, with Young’s relation, gives H = 2γcosθ eq/ρ gR = 2cosθ eqℓc 2/R. For example, if water perfectly wets a tube with radius R = 10−5 m, the capillary rise is about 1 m. Leaves have pores with radii significantly smaller than 1 µm, which helps to explain why the tallest trees can achieve heights of about 100 m.
Capillary adhesion occurs when two wetted surfaces are brought into contact. When the contact angle is smaller than 90°, the surfaces stick together strongly. Between grains of sand, for example, water forms bridges that are partially responsible for the stability of sandcastles. Some beetles use capillary adhesion to walk on surfaces and, when attacked by predators, significantly enhance their sticking ability. Another example is elastocapillarity, the ability of two flexible sheets to cling together in much the same way that hairs of a paintbrush coalesce after being withdrawn from paint. In elastocapillarity, the elastic energy of the deformed material is included in the energy minimization that determines equilibrium.
Surface active agents, or surfactants, can alter the properties of wetting and capillary action. Such molecules have two parts—a hydrophilic polar head and one or more hydrophobic aliphatic chains—whose different chemical affinities cause them to adsorb to boundaries between fluid phases similar to the water–oil interface. Surfactants are a key component of soaps and detergents because they attract polar molecules like water and nonpolar molecules like grease or oil. As a consequence of their chemical duality, surfactants, when dissolved, have their lowest energy state at the surface. Thus, in their presence, deformation of an interface requires less energy; in other words, the surface tension is reduced. A paper clip floating on clean water rapidly sinks when a drop of detergent is added.
Much remains to be said about both static and dynamic aspects of wetting, some of which is covered in the further readings and supplemental materials available with the online version of this Quick Study. If you should be drinking through a narrow straw while looking them over, remember that capillary action is doing part of the work for you and you are enjoying all the benefits!
We thank Armand Ajdari for his critical reading and helpful comments. We are especially grateful to Charles Holbrow for careful editing, suggestions, and the title.
References
1. P.-G. de Gennes, F. Brochard-Wyart, D. Quéré, Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves, A. Reisinger, trans., Springer, New York (2004).
2. D. Lohse, Physics Today, February 2003, page 36. https://doi.org/10.1063/1.1564347
3. Y. Pomeau, E. Villermaux, Physics Today, March 2006, page 39. https://doi.org/10.1063/1.2195314
More about the authors
Laurent Courbin (lcourbin@deas.harvard.edu
Laurent Courbin, 1 lcourbin@deas.harvard.edu .
Howard A. Stone, 2 has@deas.harvard.edu .




