Wickless heat pipes in microgravity
DOI: 10.1063/PT.3.3704
Heat pipes combine thermal conduction, phase change, and (at least in most designs) capillary flow to transport energy efficiently between a heat source and a heat sink. They can be used for cooling laptop microprocessors and graphics processors, keeping permafrost from thawing, and thermal management in spacecraft and satellites. Because the heat pipe’s fluid circulation is driven by interfacial forces, the devices operate without any moving parts, which makes them simple, light, and reliable.
Nonetheless, the humble heat pipe is still able to surprise when one looks at the fluid dynamics inside. In experiments conducted with a simply designed heat pipe on the International Space Station, we discovered, for example, that the pipe’s working fluid can condense at the heat-source end, where the temperature greatly exceeds the fluid’s boiling point.
Figure
Figure 1.

A heat pipe guides heat from a hot evaporator to a relatively cool condenser. It typically includes a wicking structure to help return condensed liquid to the evaporator.
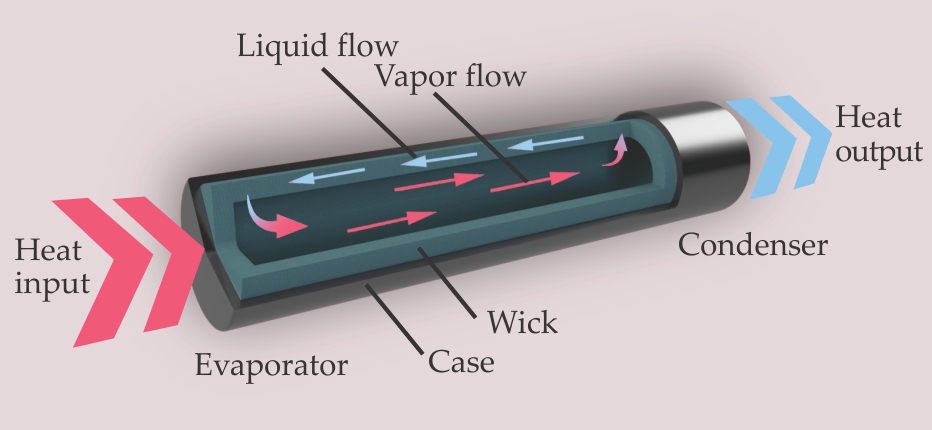
The heat pipe was invented in 1936 by Jacob Perkins. It was a gravity-assisted design, in which the lower end of the tube pointed toward the heat source, a furnace, and the higher end was directed toward a heat sink, a boiler. The first capillary-driven heat pipe was patented by Richard Gaugler of General Motors in 1945 and was independently rediscovered in 1963 by George Grover at Los Alamos National Laboratory; Grover was the one who coined the term heat pipe. NASA furthered the development of heat pipes for use in satellites, and the agency’s involvement catalyzed a commercial market for the devices. Nowadays, several million of them are produced every month worldwide. More historical and technical information can be found in the excellent text by Amir Faghri cited in the additional resources.
It could be good not to be wicked
Most heat pipes contain wicks to assist in returning liquid to the heated end, and in those designs an effective wick structure is the key to good performance. Wicks are normally made from metal meshes or sintered metal powders. Some heat pipes, though, use grooves or corners instead of a wick. Because they have no wicks to degrade or clog, those models are less complicated to manufacture, have longer working lifetimes, and are more reliable. The lighter, wickless heat pipes are particularly attractive for space applications; capillary forces need not be so large in space, and each additional kilogram can add tens of thousands of dollars to the launch cost.
Wick design is still more of an art than a science, and wick structures may be difficult to manufacture and to keep clean or intact over long periods of time. That problem is especially acute for applications such as NASA’s Journey to Mars mission, which put a premium on reliability and minimal maintenance. From an experimental point of view, complex wicking structures make it impossible to study the interfacial phenomena driving vaporization, condensation, and liquid return. Understanding the interfacial phenomena in a heat pipe is key to developing optimal designs for long-term space and terrestrial applications. Thus, the Constrained Vapor Bubble (CVB) project, in which we are participating, was initiated by researchers from Rensselaer Polytechnic Institute, supported by a science and engineering team from NASA, and run on the International Space Station.
The CVB experiment used a simple heat pipe, a bubble of pentane vapor in a glass spectrophotometer cuvette 3 mm wide. The sharp corners of the cuvette create the capillary force required to pump liquid from the condenser to the evaporator. An electrical resistance heater was attached to the evaporator end, while at the other end a set of thermoelectric coolers kept the condenser temperature fixed. Because the walls of our heat pipe are transparent, we could see the location of the liquid–vapor interface and study the fluid dynamics. In particular, as shown in figure
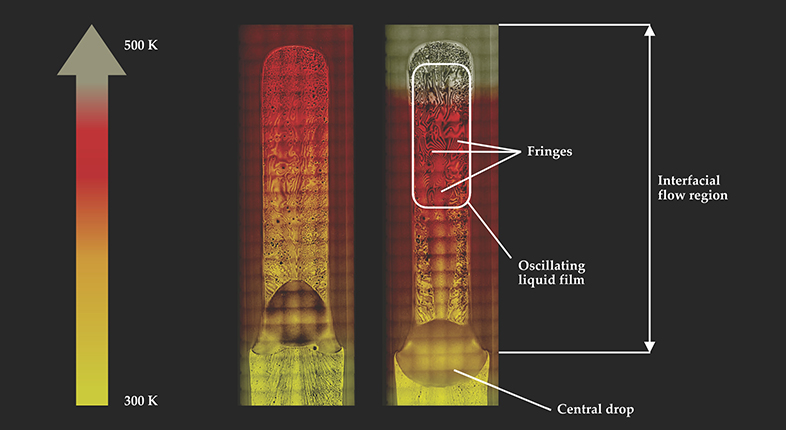
Figure 2.

Surface condensation on the walls of a heat pipe is revealed by interference fringes. Shown here is the pipe’s interfacial flow region, which extends from the hot evaporator at the top of the device to a central drop. (The condenser of the heat pipe, well below the central drop, is not shown.) The density of fringes, and thus the amount of surface condensation, is greater near the evaporator end of the hotter, right-hand heat pipe than it is near the end of the relatively cool left-hand pipe. The background grid structure arises because the figures are composites, stitched together from many individual frames.
Flooding, a new performance limitation
In the microgravity environment of the International Space Station, the two main interfacial forces governing fluid flow inside a wickless heat pipe are the capillary and Marangoni forces. When the amount of liquid evaporating is larger than what can be pumped by the capillary force, the evaporator end of the heat pipe begins to dry out. That “capillary limit” is the most common performance limitation of a heat pipe. The Marangoni force causes liquid to flow in response to a change in its surface tension. In a heat pipe filled with a pure fluid, the Marangoni force drives the liquid from the evaporator end, where the temperature is high and the surface tension is generally low, to the condenser end, where the temperature is low and the surface tension is high. The flow due to Marangoni forces opposes the flow generated by capillary forces, so Marangoni forces can cause the evaporator to dry out even before the capillary limit is reached.
Many groups have reported on the capillary limit in terrestrial experiments. The CVB group, however, was the first to visualize the complete vapor–liquid interface in a heat pipe, and we found that the heat pipe would not actually dry out. Instead, the evaporator end flooded. In fact, it filled with ever more liquid as we increased the heater power. As the flooded region grew, the pipe did a poorer job of transferring heat, just as would happen if the heater were drying out.
Figure
Condensation near the heater end
Condensation normally occurs on cooled surfaces, but it can occur at temperatures higher than a liquid’s normal boiling point in regions of high interface curvature, such as around small particles that nucleate raindrops or in tight crevices like those on the soles of gecko feet. In the CVB experiment, we found that liquid condensed in the interfacial flow region even at temperatures 160 °C above pentane’s boiling point of 36 °C. Furthermore, as we supplied more heat, the condensed liquid film became thicker.
The liquid nearest the heater was unstable in that its thickness periodically changed over time; with increased heat input, the amplitude and frequency of the oscillations also increased. Those oscillations, driven by the large Marangoni force in the region near the heater end, provided the regions of high curvature and strong intermolecular forces required for condensation at an anomalously high temperature. Marangoni and capillary forces are also important for terrestrial heat pipes, but gravity’s pull on the liquid is usually stronger. As a result, the effects of the Marangoni force are disguised and can easily be falsely attributed to surface contamination.
Our investigation of wickless heat pipes in a microgravity environment has revealed new, counterintuitive phenomena. Experimental and theoretical work is continuing as we and others try to better understand the origins of those phenomena and use that knowledge to design improved devices.
References
► A. Faghri, Heat Pipe Science and Technology, 2nd ed., Global Digital Press (2016).
► A. Kundan et al., “Thermocapillary phenomena and performance limitations of a wickless heat pipe in microgravity,” Phys. Rev. Lett. 114, 146105 (2015). https://doi.org/10.1103/PhysRevLett.114.146105
► A. Kundan et al., “Condensation on highly superheated surfaces: Unstable thin films in a wickless heat pipe,” Phys. Rev. Lett. 118, 094501 (2017). https://doi.org/10.1103/PhysRevLett.118.094501
More about the authors
Joel Plawsky is a professor of chemical engineering and Thao Nguyen is a postdoctoral research fellow, both at Rensselaer Polytechnic Institute in Troy, New York.






