Mimicking mussels in the lab
DOI: 10.1063/PT.3.5128
Human beings use more than 6 billion pounds of glues and adhesive tapes every year. Widely applied in the automotive, aerospace, furniture, electronics, and construction industries, they are pervasive. Unfortunately, most of them are not degradable; many pile up in landfills, and some even release toxic gases into the atmosphere.
Adhesives have become indispensable in sealing tissues and dressing wounds after surgical procedures. Yet water makes up 60% of our bodies, and most synthetic adhesives cannot bind tightly to wet surfaces. Physicians must usually dry a wound before applying a bandage. To stick to any surface, those adhesives rely on a combination of ionic and van der Waals interactions with surface elements and on mechanical interlocking. Yet the large dielectric constant of water compromises the bonds made from those interactions; water molecules bind tightly to surfaces and prevent adhesives from displacing them.
To mitigate such problems and design better-performing adhesives, scientists have taken inspiration from nature. Many underwater creatures such as mussels, barnacles, sandcastle worms, and octopuses use different strategies to bind themselves to surfaces in an aquatic environment. Although biologists have only begun to study how some of those organisms perform their underwater magic, they have attempted to mimic the adhesive strategies of mussels for more than two decades.
A protein’s sticky solution
Ordinarily, marine mussels cling to rocky shorelines in turbulent intertidal zones, as shown in figure
Figure 1.

Mussels bound to the shoreline. The animals’ adhesive proteins contain 3,4-dihydroxyphenylalanine, or DOPA (red), and the adhesive molecule catechol. Incorporating catechol is an oft-used strategy to create a synthetic adhesive for binding to wet surfaces. (Photo by Matthew Fox Photography/Shutterstock.com.)

To attach to a surface, mussels secrete liquid proteins that solidify within seconds to form surface-bound adhesive plaques. Those proteins are made of the uniquely modified amino acid 3,4-dihydroxyphenylalanine, or DOPA. The amino acid contains a catechol group—a benzene ring with two hydroxyl groups attached to adjacent carbon atoms—that has two main purposes. It forms strong bonds with a surface and quickly solidifies the adhesive proteins through chemical cross-linking.
The adhesive chemistry of catechol underlies its ability to form strong bonds with metals, polymers, and even biological tissues. That chemistry is quite diverse: Catechol can form either permanent (covalent) bonds; reversible bonds, such as cation–π interactions with positively charged surfaces; or hydrogen bonds with polar molecules on surfaces. And when catechol is oxidized—either chemically or enzymatically—it transforms into its highly reactive form, known as quinone. In turn, quinone can cross-link with another catechol molecule that cures the adhesive proteins.
Nurture over nature
Although it is feasible to directly harvest those adhesive proteins from mussels, it would take more than 10 000 of the animals to produce a mere gram of the protein. That makes scaling up the natural production of the adhesive difficult and impractical. Instead, most scientists focus on synthetic approaches to produce it.
When catechol is chemically attached to a polymer intended as one of those mussel mimics, it makes the polymer sticky. Fortunately, it’s possible to tune the polymer’s composition—and thus control its adhesive strength, degradation rate, and biocompatibility. The simplicity and versatility of that approach enable materials scientists to design adhesives for a variety of applications, including stopping bleeding, repairing tendons, and sealing incisions usually closed by sutures, staples, and tacks.
Part of catechol’s lure is its ability to bind to metal and metal ions. Scientists can sequester antimicrobial nanoparticles and silver ions into the adhesive matrix, where they are released over time to prevent infection. What’s more, reactive oxygen species (ROS) such as hydrogen peroxide are generated as by-products of the oxidation of catechol.
Those ROS generated from catechol turn out to be antimicrobial and antiviral. That’s appealing because our bodies generate ROS as part of their normal immune response, and ROS naturally degrade into water and oxygen. Alternatively, one can chemically modify catechol using a halide atom such as chlorine, bromine, or iodine. Researchers have found that such halogenated catechols can kill many strains of antibiotic-resistant bacteria.
On-off switches
Natural mussel proteins attach to a surface permanently. But the adhesive characteristics of catechol, more generally, respond when the pH of any liquid surrounding it changes or when catechol is exposed to an electric current. For instance, forcing an electric current through a catechol-containing adhesive that is already attached to a surface prompts the adhesive to detach.
That switchability arises from the adhesion difference between the natural form of catechol and its oxidized form, quinone. The latter is only 20% as adhesive as catechol. And by controlling its state using pH or electrochemical oxidation, one can effectively tune the adhesion. But the reactive nature of quinone also renders it unsuitable for repeated bonding. Fortunately, a protective chemical group of boronic acid prevents the oxidative cross-linking of catechol and can preserve its reversibility in redox reactions.
In 2021 Conghui Yuan and Lizong Dai (both polymer scientists at Xiamen University in China) and their collaborators integrated a catechol-containing polymer—a hydrogel in this case—onto the feet of a 50-gram climbing robot, pictured schematically in figure
Figure 2.

A climbing robot in action. (a) With electric pads made of adhesive hydrogel as its feet, the robot adheres to a vertical, conductive metal surface. When a current runs through the adhesive, it becomes bound to the metal surface. The inset shows the chemical structure of bound catechol. (b) Reversing the current—so that anode and cathode switch places—weakens the adhesive enough for the robot’s foot to detach. The black arrows indicate its direction of movement. The inset shows the chemical-structure change. (Adapted from B. P. Lee, Sci. Robot. 6, eabh2682, 2021, doi:10.1126/scirobotics.abh2682
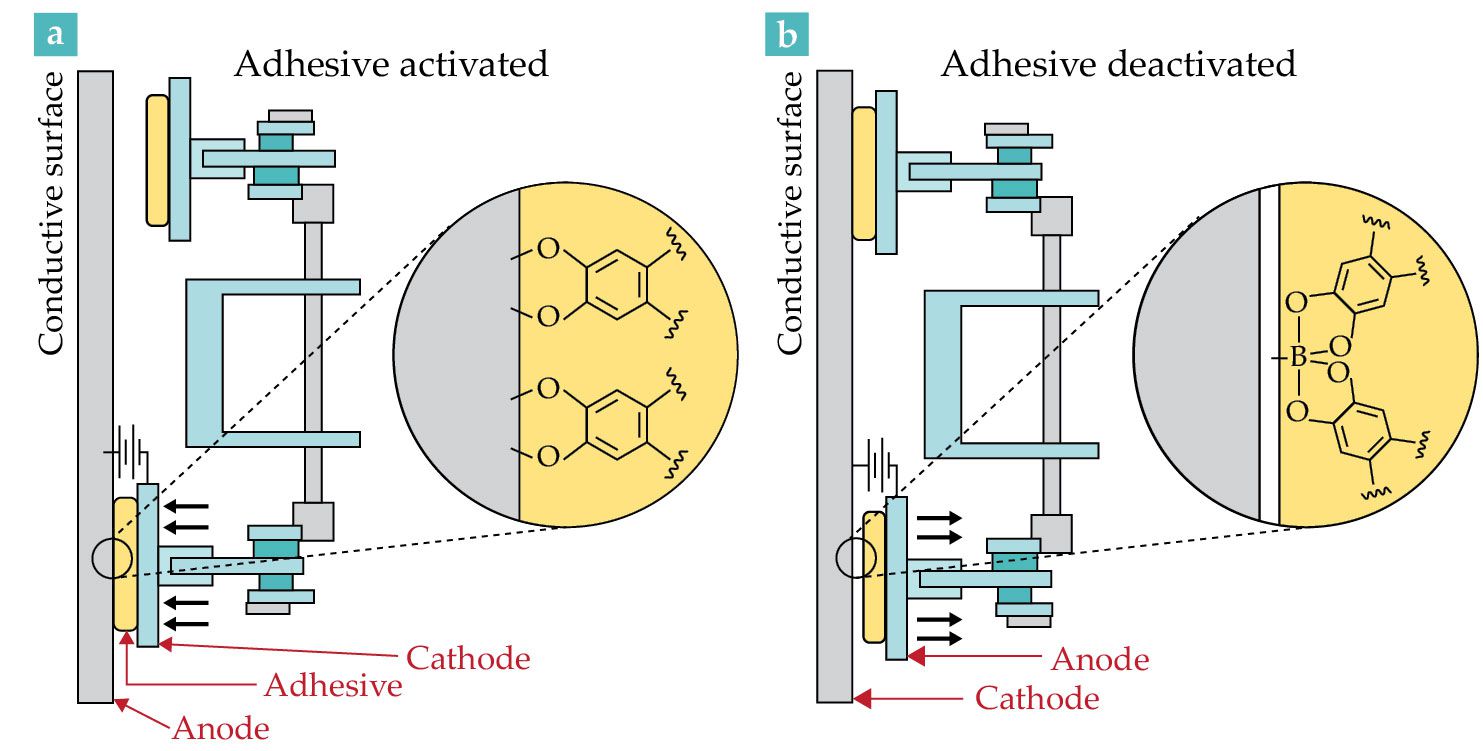
Deactivating bound adhesives would also be an appealing option for physicians removing wound dressings. It would minimize pain and avoid damaging skin. Moreover, the ability to deactivate an adhesive on command would allow scientists to more easily disassemble bonded components prior to recycling them.
Currently, catechol chemistry is mostly used to design mussel-mimetic adhesives that bind to wet surfaces. But those mimics pale in comparison with the performance and complexity of natural proteins created by the mussels themselves. The animals rely on many types of proteins to construct adhesive plaque, with each protein having a different function. Some work as a surface primer, some provide mechanical strength, and others offer a protective coating.
Additionally, mussels are tethered to their surface-bound plaque via stretchable “byssal” threads that act as shock absorbers and soften the impact when the mussels are repeatedly pulled by pounding waves. That integrated design is incredibly difficult to replicate and exploit. Chemists and materials scientists have a long way to go to fully duplicate the strong underwater adhesion of mussels. But they’re working on it.
References
▶ W. Zhang et al., “Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications,” Chem. Soc. Rev. 49, 433 (2020). https://doi.org/10.1039/C9CS00285E
▶ H. M. Siebert, J. J. Wilker, “Deriving commercial level adhesive performance from a bio-based mussel mimetic polymer,” ACS Sustainable Chem. Eng. 7, 13315 (2019). https://doi.org/10.1021/acssuschemeng.9b02547
▶ B. Liu et al., “Antimicrobial property of halogenated catechols,” Chem. Eng. J. 403, 126340 (2021). https://doi.org/10.1016/j.cej.2020.126340
▶ M. S. A. Bhuiyan et al., “In situ deactivation of catechol-containing adhesive using electrochemistry,” J. Am. Chem. Soc. 142, 4631 (2020). https://doi.org/10.1021/jacs.9b11266
▶ J. Huang et al., “Electrically programmable adhesive hydrogels for climbing robots,” Sci. Robot. 6, eabe1858 (2021). https://doi.org/10.1126/scirobotics.abe1858
More about the authors
Bruce Lee is a professor of biomedical engineering at Michigan Technological University.





