Jammed particles, from sandy beaches to sunscreens
DOI: 10.1063/1.3518220
Mayonnaise, sandstone, shaving cream, and frozen peas have something in common: They are all types of jammed matter, a class made of randomly packed particles. Mayonnaise, for example, is made of oil droplets in water; sandstone, of grains; and shaving cream, of bubbles. Schoolchildren may be taught that solids, liquids, and gases are the fundamental phases of matter, but a new phase—jammed matter—emerges when particles pack densely enough that they all touch their neighbors and resist flow. If the particles are small enough that they can rearrange due to thermal fluctuations, then their structural arrest can be captured by the nonequilibrium physics of glassy systems. However, the physics of the jamming transition remains elusive for systems of larger particles that are insensitive to temperature. Those nonequilibrium, disordered systems are currently of great interest to engineers and physicists alike.
Jammed matter at home
A simple way to create jammed matter in the kitchen is to fill a cup with sugar and make a static packing. Strictly speaking, the process does not yield a rigid packing: A few of the sugar grains can still rattle and roll if you shake or tap the cup. In fact, experiments have shown that the recipe for creating jammed matter is, as James Bond would say, “shaken, not stirred.” Gently shaking or tapping the cup settles the rattling particles and removes any voids created by the packing protocol. Vigorous stirring, on the other hand, would actually introduce additional voids.
In general, a jammed state results when the density is large enough that the packed particles can support their own weight and resist mechanical shear; that is, they resist up to a point called the yield stress. If a stress greater than the yield stress is applied to a jammed system, the system will unjam and its particles will flow. The nature of the jamming transition depends on whether the particles are rigid or soft, smooth or rough—and also on how the packing is created. Sedimentation under gravity, for example, will lead to a different packing structure than will isotropic compression. (For more details, see the article by Anita Mehta, Gary Barker, and Jean-Marc Luck in Physics Today, May 2009, page 40
Nevertheless, experiments have demonstrated that packing and shaking hard spheres of a fixed size—glass beads, ball bearings, and green peas, for example—reproducibly give a packing in which, to within experimental error, 64% of the volume is filled. The universality of the result has inspired the term “random close packing,” even though no ab initio theory can explain how RCP comes about. Still, the phenomenon suggests a general principle of organization for randomly arranged particles, a prospect that puts a gleam in the eyes of physicists and sends them hurriedly to work.
A matter of particle particularities
Experimental and numerical studies have revealed disordered packing structures for nonspherical particles such as tetrahedra or ellipsoids, for otherwise identical spheres with varying degrees of friction, and for systems of spheres with a broad distribution of sizes. Deformable spheres or shapes that can pack tighter than spheres push the jamming threshold to densities greater than the 64% RCP value; forces due to attraction or friction allow for looser jammed structures. For example, rough sugar grains with varying shapes pack less tightly than the smooth, deformable spheres found in emulsion droplets in butter, mayonnaise, and beauty lotions.
To learn about the geometries associated with different types of packed particles, scientists measure specific characteristics for every grain in the pack. For instance, we can measure the local particle density or we can count the number of neighbors surrounding each particle and so probe the local geometry. The number of contacts a particle has with its neighbors provides an assessment of the system’s rigidity. The statistics of such local features distinguish between packings. And if researchers are lucky, they will also reveal unifying features indicative of physical laws that explain why many experiments yield the same or similar distributions.
An inside job
A significant challenge for those of us investigating jammed matter is that the particles inside the packing are hidden from view. In the very first study, now 50 years old, of the structure of jammed matter, John Bernal and J. Mason laboriously picked apart ball bearings one by one and noted by hand the positions and contacts between them. These days, researchers are examining jammed materials with x-ray tomography and magnetic resonance imaging—two techniques commonly used in medicine to see through the body—in combination with sophisticated image-analysis algorithms.
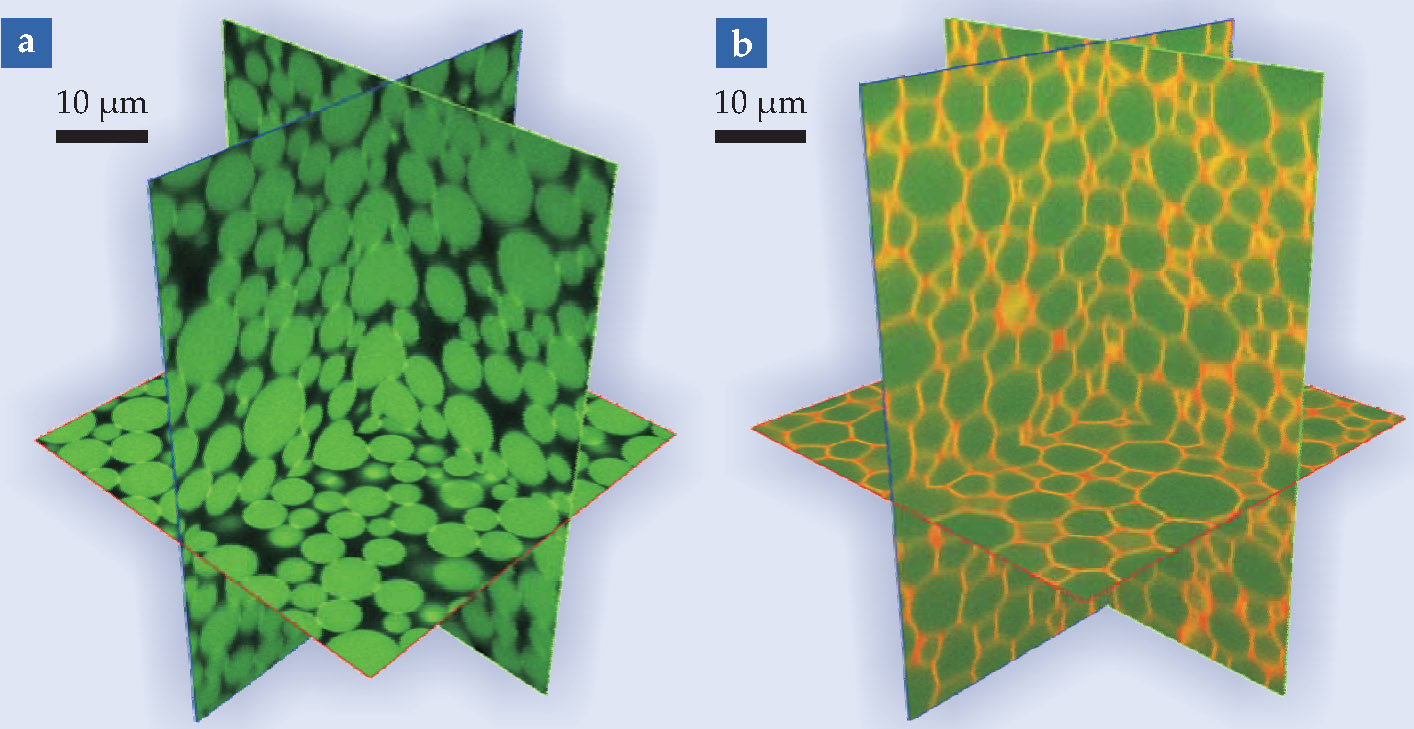
To investigate the packing of emulsion droplets, gels, and pastes made of micron-sized synthetic particles or corn starch, our research group at New York University uses visible-light microscopy. We render the packing transparent by matching the refractive index of the particles to that of the fluid in which they are suspended; then we apply a fluorescent dye and observe the particles through a confocal microscope. The figure displays examples of three-dimensional packing structures obtained with our method. At present, we can image 1000 particles in a minute. It takes about an hour with an ordinary desktop computer to analyze the particles’ packing geometry, as revealed by the distributions of neighbor number and particle density.

Packings under the microscope. These confocal microscopy images show fluorescent emulsion droplets whose radii vary in size from about 2 to 5 µm. (a) Attractive droplets, such as often found in ice cream, stick together when packed. (b) A compressed emulsion forms a foam that looks like the detergent solution shown in the May 2010 Quick Study by Doug Durian and Srini Raghavan (page 62). Here the green and orange represent silicone oil and water, respectively.
(Images courtesy of Ivane Jorjadze and Lea-Laetitia Pontani, New York University.)
One of the first exciting discoveries about systems of jammed particles was that their geometric structure is indistinguishable from that of liquids. That equivalence presents a puzzle: Jammed matter has the geometry of a liquid and the rigidity of a solid. You can take a stroll on a sandy beach, but only a select few have been known to walk on water. How can that be?
The solution rests on a third important quantity mentioned above—the number of contacts a particle has with its neighbors. Particles in a liquid can rearrange freely, but jammed matter is stuck by close contacts. When the field was in its infancy, scientists would determine contact distributions by pouring wax over the packing and identifying inaccessible regions. Nowadays we have cleverer methods—for example, labeling particles with dyes that fluoresce to indicate where the particles are in contact.
Straightforward Newtonian force balance requires that for packed smooth spheres, the average number of contacts must be at least six. (The general restriction for d dimensions is that the number of contacts be at least 2d.) That result, derived by James Clerk Maxwell in the 19th century, has been experimentally confirmed. It is the way in which the contact number fluctuates locally throughout the packing that reveals a piece of the mystery of packing. Recent experiments have shown that a mix of particles of different sizes gives a different contact-number distribution than for a system of same-sized particles, but the global constraint on the average number of contacts always holds.
Other techniques reveal not just the number of contacts, but also contact forces. Those may involve deforming particles away from their relaxed spherical shape or using photoelastic beads. (See the Quick Study by Jackie Krim and Bob Behringer in Physics Today, September 2009, page 66
A physics of jammed matter?
Measurements of the local neighborhood of each particle in a jammed system and of the forces acting on the particles specify the state of the jammed material. The ability to make those measurements has raised a number of questions that have been addressed by theoretical models for how particles arrange themselves to yield specific contact and other distributions. A first-principles approach to explaining jammed matter would identify the physical origin of the randomness in packings and predict the distributions of contacts, number of neighbors, forces, and densities. To date, nobody has devised a parameter-free model that captures physical reality, though some have enjoyed success with more modest efforts that include empirical parameters.
Higher-level theories would interpret the randomness in packings in terms of a more general organizing principle, just as thermodynamics encapsulates thermal fluctuations via the temperature. More than 20 years ago, Samuel F. Edwards conjectured a statistical mechanics framework for jammed matter, in which the volume of the packing corresponds to the energy of thermal equilibrium systems. The theory predicts a “compactivity” analogous to temperature. But whereas thermometers are common, a compactometer to measure compactivity has yet to be invented. Intuitively, the fluffier the packing, the easier it is to compact; hence it has higher compactivity. At a given compactivity, the packing will minimize the total volume and therefore reach the densest random packing. The theory gives one way to explain the celebrated 64% density ubiquitously obtained for the RCP of single-sized spheres, but it also assumes that concepts from equilibrium statistical mechanics can be translated to nonequilibrium jammed systems. Time will tell whether any of the current theories hold up. Meanwhile, experimental and theoretical work continues apace in the exciting field of jammed matter.
References
1. J. D. Bernal, J. Mason, “Packing of Spheres: Co-ordination of Randomly Packed Spheres,” Nature 188, 910 (1960).
2. S. F. Edwards, R.B. S. Oakeshott, “Theory of Powders,” Physica A 157, 1080 (1989).
3. A. J. Liu, S. R. Nagel, “Nonlinear Dynamics: Jamming Is Not Just Cool Any More,” Nature 396, 21 (1998).
4. J. Brujic et al. ., “3D Bulk Measurements of the Force Distribution in a Compressed Emulsion System,” Faraday Discuss. 123, 207 (2003).
5. M. Clusel et al. ., “A ‘Granocentric’ Model for Random Packing of Jammed Emulsions,” Nature 460, 611 (2009).
More about the authors
Jasna Brujic is an assistant professor of physics at New York University in New York.
Jasna Brujic, New York University, New York, US .




