A physicist’s tour of the upper atmosphere
DOI: 10.1063/1.3047701
The air at an altitude of 50 km is one-thousandth as dense as that at Earth’s surface. Moreover, the density of Earth’s atmosphere decreases exponentially with height, so the upper atmosphere—the region between 50 and 1500 km above the surface—includes less than 1% of the total atmospheric mass. Nonetheless, the upper atmosphere is a buffer against the harsh conditions of space, able to absorb energetic particles and solar radiation even more biologically damaging than that which the lower-lying ozone layer intercepts. As a partially ionized plasma, the upper atmosphere interacts strongly with radio signals that are beamed through it to communicate with orbiting satellites. When the plasma is turbulent, for example, global positioning system signals can become unusable. Beyond the upper atmosphere is space, including the magnetosphere, where the solar wind interacts with Earth’s magnetic field. Many of the processes that govern the lower atmosphere have prominent roles in the upper atmosphere. But the density, composition, and energy sources of the upper atmosphere cause it to behave quite differently.
Composition and temperature
Whereas the lower atmosphere is vertically mixed by turbulent diffusion, the upper atmosphere above about 100 km is sufficiently thin that molecular diffusion dominates. Lighter species separate and drift upward through the heavier species to create stratified composition regions. In contrast, the lower atmosphere has a vertically uniform composition of its major species.
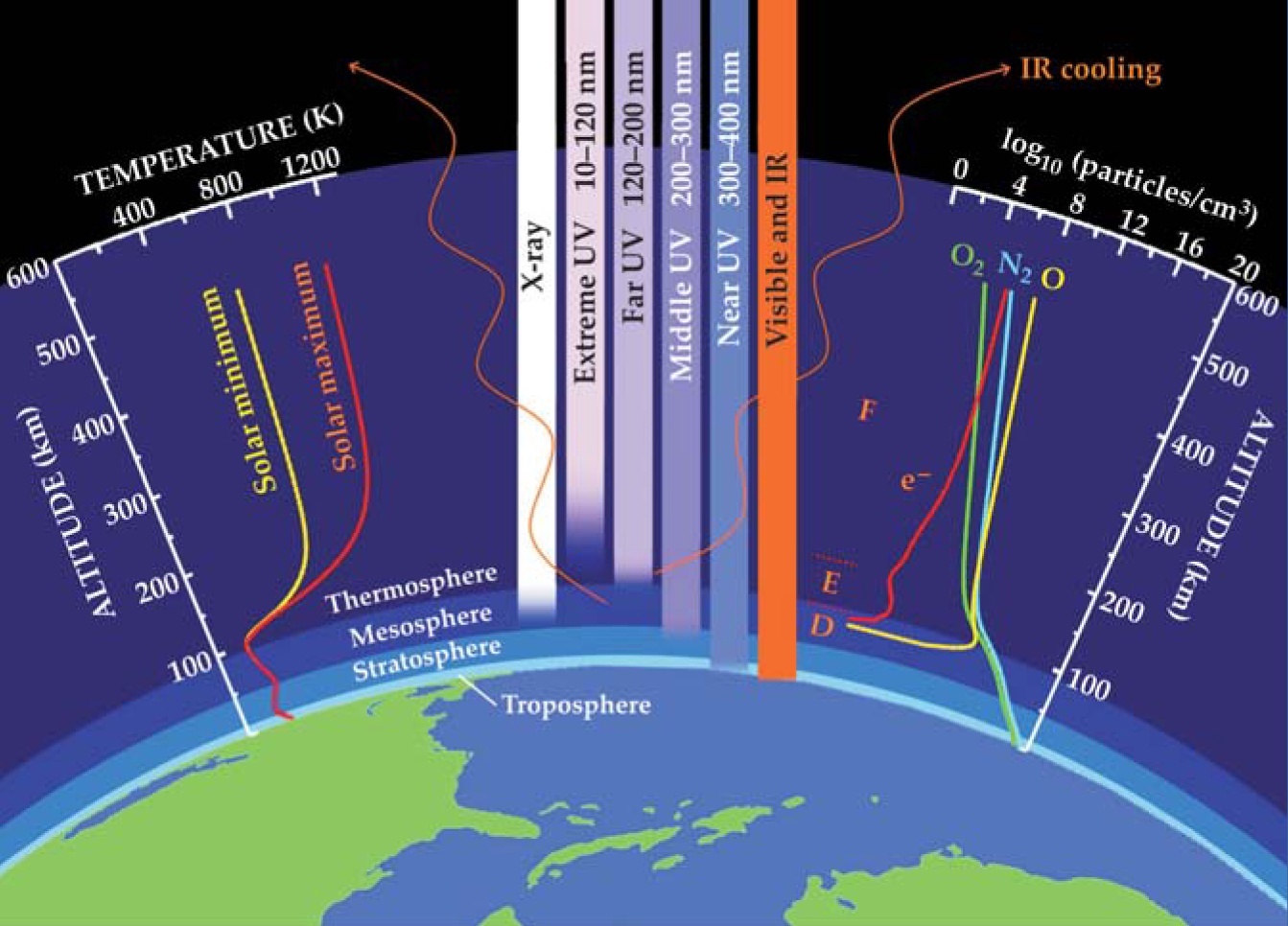
Photochemistry further shapes the composition of the upper atmosphere. UV solar radiation at wavelengths shorter than 200 nm dissociates oxygen (O2) and creates a large population of monatomic O, whose diffusive separation makes it the dominant species between 200 and 600 km. The figure illustrates the diffusive separation with a plot of the O density and that of O2 and nitrogen (N2), the two other major species in the 200–600 km region. The presence of highly reactive O greatly increases the chemical complexity of the fluid, permitting the formation of important chemical products such as monatomic hydrogen, hydroxyl, nitric oxide, and ozone. In addition, the ceaseless influx of disintegrating meteors creates a trace population of metals, primarily iron and sodium, between 80 and 105 km.
Solar radiation at wavelengths shorter than about 100 nm liberates electrons and turns most of the upper atmosphere into a partially ionized plasma called the ionosphere. Its major ion species are O+, O2 +, and NO+. Below 100 km, ion density diminishes rapidly due to the dwindling supply of ionizing radiation and increasingly rapid recombination at higher pressures. Above 400 km, diffusion processes dominate and ion density falls off exponentially but less rapidly than the density of neutral species. In between, as shown on the figure, there is a density peak near 300 km and a smaller one near 110 km; the areas surrounding those peaks are the F and E regions, respectively. Extending from about 60 km to 95 km, the D region is a poorly understood chemical mix of positive, negative, and cluster ions. At night, the D region essentially vanishes and the E region’s ionization rapidly decays, but the F region persists due to slow recombination rates and a supply of ionization from above the upper atmosphere.
Radiative heat transfer and collisional heat transfer, along with diffusive transport and chemical reactions, determine the basic thermal structure of the upper atmosphere. Absorption of solar UV radiation is the primary heat source; shorter-wavelength photons generally deposit their energy at higher altitudes. An essential difference between the lower atmosphere and upper atmosphere is that the heating and cooling processes in the upper atmosphere are far removed from local thermodynamic equilibrium. Energy is partitioned among ionization, molecular dissociation, internal atomic and molecular excitation, and direct heating. The chemical potential energy of dissociated molecules and ions can be transported far from its source before being converted to thermal energy. Much of the energy that produces internal excitation is radiated as airglow at various wavelengths from IR to extreme UV.
The primary mechanism by which the upper atmosphere is cooled involves IR emission by carbon dioxide (mainly below 120 km) and NO (mainly at or near 150 km). Heat deposited above 150 km is thermally conducted downward, transferred via collisions to excited states of those cooling agents, emitted as IR radiation, and lost to space. Cooling is inefficient at higher altitudes because the major species there—O, O2, and N2—do not radiate efficiently in the IR. Consequently, the upper atmosphere above 150 km is very warm, 600–1400 K. That region and the region of strong temperature gradients between 90 and 150 km together compose the appropriately termed thermosphere. The mesosphere is defined by a minimum temperature of about 180 K near 90 km and a local maximum of 260 K near 50 km that results from middle-UV absorption by O3 (see the figure). Because of its efficient cooling processes, the upper mesosphere is the coldest part of the atmosphere. The summer polar mesosphere is so cold that despite its very low pressure and concentration of water, H2O crystallizes into ice to form the atmosphere’s highest layer of clouds (see the Back Scatter photo in June 2007, page 92

Thermal and compositional structure of the atmosphere. The upper atmosphere, comprising the mesosphere, thermosphere, and embedded ionosphere, absorbs all incident solar radiation at wavelengths less than 200 nm. Most of that absorbed radiation is ultimately returned to space via IR emissions. The stratospheric ozone layer absorbs radiation between 200 and 300 nm. The plot on the left shows the typical global-average thermal structure of the atmosphere when the flux of solar radiation is at the minimum and maximum values of its 11-year cycle. The plot on the right shows the density of nitrogen (N2), oxygen (O2), and monatomic oxygen (O), the three major neutral species in the upper atmosphere, along with the free electron (e−) density, which is equal to the combined density of the various ion species. The F, E, and D regions of the ionosphere are also indicated, as is the troposphere, the atmosphere’s lowest region.
Dynamics
The horizontal structure of the upper atmosphere is influenced both by the spatial distribution of its heat sources and by large-scale circulation, which provides horizontal transport of compositional and thermal variations. The spatial distribution of solar heating, the chief driver of upper-atmosphere dynamics, produces horizontal temperature and pressure gradients and a consequent system of winds. Because of Earth’s rotation, those patterns generally migrate westward with the Sun and form a diurnal oscillation. The Sun also influences horizontal dynamics through other atmospheric tides—oscillations with an integral number of periods in a solar day. Planetary waves, which are global-scale oscillations with periods greater than a day, are also drivers of horizontal dynamics. Some tidal and planetary waves are excited in situ; others propagate upward from lower altitudes.
Many important electrodynamical processes derive from large-scale circulation. Ions and electrons respond differently to neutral-species flows because of differing ion–neutral and electron–neutral collision frequencies. Winds thereby provide an electromotive force that generates systems of currents and electric fields. Those dynamo systems are very complex, due to the anisotropy—oriented by the geomagnetic field—of upper-atmosphere conductivity and the conductivity’s strong dependence on height and local time. A prominent consequence is the equatorial plasma fountain: A wind-generated electric field in the equatorial region raises both ions and electrons several hundred kilometers, whence they diffuse to lower altitudes and higher latitudes along magnetic field lines.
In addition to internally generated electric fields, highly variable electric fields are imposed on the upper atmosphere by the magnetosphere. Those fields are particularly influential at high latitudes, where they propel plasma circulations to typical speeds of 500–1500 m/s. The high-speed ions in turn spin up the neutrals to speeds of 200–600 m/s; the difference between the ion and neutral speeds results in frictional heating. Also at high latitudes, energetic particles from the magnetosphere precipitate along magnetic field lines into the upper atmosphere, where they deposit much of their energy and spawn the aurora (see the Quick Study by Bob Strange-way, July 2008, page 68
Atmospheric gravity waves, also called buoyancy waves, are key dynamical features and a source of much uncertainty in geophysicists’ understanding of upper-atmosphere behavior. Excited in both the upper atmosphere and lower atmosphere—for example, by airflow over mountains—gravity waves have horizontal wavelengths of 10-1000 km and propagate into and through the upper atmosphere (see the Back Scatter photo in June 2006, page 96
Upper-atmosphere processes interact in tangled and poorly understood ways. Furthermore, the diverse energy and momentum inputs into the upper atmosphere vary on a broad range of time scales. Solar variations and sporadic energy outbursts are particularly influential (see the article by Judith Lean, June 2005, page 32
The online version of this Quick Study includes further resources and links to captioned illustrations of upper-atmosphere phenomena.
References
1. G. W. Prölss, Physics of the Earth’s Space Environment: An Introduction, Springer, New York (2004).
2. R. W. Schunk, A. F. Nagy Ionospheres: Physics, Plasma Physics, and Chemistry, Cambridge U. Press, New York (2004).
More about the authors
John T. Emmert, US Naval Research Laboratory, Washington, DC, US .




